Page 150 - Geochemistry of Oil Field Waters
P. 150
138 INORGANIC CONSTITUENTS AND PHYSICAL PROPERTIES
500F --/
200t- /
0 - Nor ma1 evaoor it e
associated bine
I- /"
SODIUM, mg/l
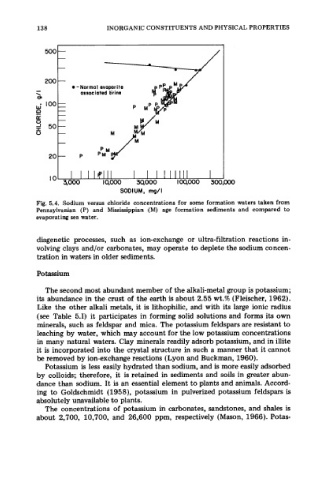
Fig. 5.4. Sodium versus chloride concentrations for some formation waters taken from
Pennsylvanian (P) and Mississippian (M) age formation sediments and compared to
evaporating sea water.
diagenetic processes, such as ion-exchange or ultra-filtration reactions in-
volving clays and/or carbonates, may operate to deplete the sodium concen-
tration in waters in older sediments.
Potassium
The second most abundant member of the alkali-metal group is potassium;
its abundance in the crust of the earth is about 2.55 wt.% (Fleischer, 1962).
Like the other alkali metals, it is lithophilic, and with its large ionic radius
(see Table 5.1) it participates in forming solid solutions and forms its own
minerals, such as feldspar and mica. The potassium feldspars are resistant to
leaching by water, which may account for the low potassium concentrations
in many natural waters. Clay minerals readily adsorb potassium, and in illite
it is incorporated into the crystal structure in such a manner that it cannot
be removed by ion-exchange reactions (Lyon and Buckman, 1960).
Potassium is less easily hydrated than sodium, and is more easily adsorbed
by colloids; therefore, it is retained in sediments and soils in greater abun-
dance than sodium. It is an essential element to plants and animals. Accord-
ing to Gol&chmidt (1958), potassium in pulverized potassium feldspars is
absolutely unavailable to plants.
The concentrations of potassium in carbonates, sandstones, and shales is
about 2,700, 10,700, and 26,600 ppm, respectively (Mason, 1966). Potas-