Page 149 - Geochemistry of Oil Field Waters
P. 149
SODIUM 137
Sodium in solution tends to stay in solution; it does not readily precipi-
tate with an anion, and it is less easily adsorbed by clay minerals than are
cesium, rubidium, potassium, lithium, barium, and magnesium. The major
source of sodium in sea water can be attributed to the weathering of rocks.
Some sodium probably was derived through volcanic activity. The ocean and
evaporite sediments contain the bulk of the sodium. Igneous rocks contain
appreciably more sodium than sedimentary rocks with the exception of
evaporites.
Sea water contains about 11,000 mg/l of sodium, as illustrated in Table
5.11. The concentration of sodium increases in brine as it evaporates, to
about 140,000 mg/l, when halite precipitates. Most oilfield waters contain
more sodium than any other cation, and most oilfield waters are believed to
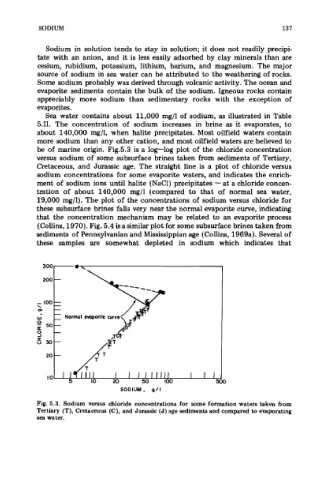
be of marine origin. Fig.5.3 is a log-log plot of the chloride concentration
versus sodium of some subsurface brines taken from sediments of Tertiary,
Cretaceous, and Jurassic age. The straight line is a plot of chloride versus
sodium concentrations for some evaporite waters, and indicates the enrich-
ment of sodium ions until halite (NaC1) precipitates - at a chloride concen-
tration of about 140,000 mg/l (compared to that of normal sea water,
19,000 mg/l). The plot of the concentrations of sodium versus chloride for
these subsurface brines falls very near the normal evaporite curve, indicating
that the concentration mechanism may be related to an evaporite process
(Collins, 1970). Fig. 5.4 is a similar plot for some subsurface brines taken from
sediments of Pennsylvanian and Mississippian age (Collins, 1969a). Several of
these samples are somewhat depleted in sodium which indicates that
SODIUM, g/l
Fig. 5.3. Sodium versus chloride concentrations for some formation waters taken from
Tertiary (T), Cretaceous (C), and Jurassic (J) zge sediments and compared to evaporating
sea water.