Page 154 - Geochemistry of Oil Field Waters
P. 154
142 INORGANIC CONSTITUENTS AND PHYSICAL PROPERTIES
water is about 5 x lo-' mg/l, and some subsurface brines contain 0.02-4.2
mg/l. Since beryllium is highly toxic, waters containing it should be handled
with caution.
Magnesium
One of the more abundant members of the alkaline earth group of metals,
magnesium makes up about 2.1 wt.% (Fleischer, 1962) of the crust of the
earth.
Magnesium is dissolved during chemical weathering, mainly as the chloride
and sulfate. Ferromagnesian minerals in igneous rocks and magnesium car-
bonate in carbonate rocks are generally considered to be the principal
sources of magnesium in natural waters. Carbon dioxide plays an important
role in the dissolution of magnesium from silicate and carbonate minerals.
Waters associated with either granite or siliceous sand may contain less than
5 mg/l of magnesium, whereas those associated with either dolomite or
limestone may contain over 2,000 mg/l of magnesium.
Elements commonly found in oilfield waters have the following ionic
potentials: sodium, 0.95; calcium, 0.50; magnesium, 0.33; chlorine, 1.81;
bromine, 1.95; and iodine, 2.16. Apparently the cation (magnesium) and the
anion (chlorine) would be the most likely to remain in true ionic solution;
however, several other variables occur during diagenesis which lead to deple-
tion of magnesium in waters.
Depletion of magnesium in some waters probably is a result of the replace-
ment reaction to form dolomite, CaMg(C0, )2. Whole mountain masses are
made of dolomite, which is formed by the regular substitution in the calcite
2oo t C
/
J
?$
Normal evaporite curve
Normal evaporite curve
'so0 500 rpoo 2,000 5ooO lop00 20,m 5Q(
MAGNESIUM, mg I I I
mg
I
MAGNESIUM,
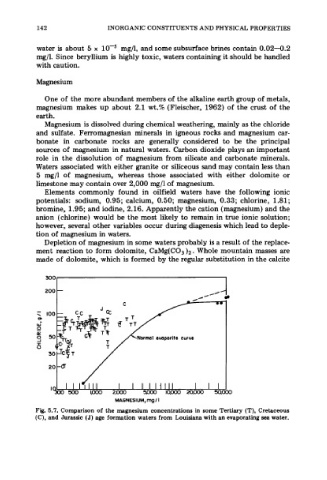
Fig. 5.7. Comparison of the magnesium concentrations in some Tertiary (T), Cretaceous
(C), and Jurassic (J) age formation waters from Louisiana with an evaporating sea water.