Page 176 - Geochemistry of Oil Field Waters
P. 176
BROMINE 163
Braitsch and Herrmann (1963) found that the absolute bromide content of
rocks can be used to determine primary and secondary paragenesis. Distribu-
tion of bromide between solution and crystals of halite, sylvite, carnallite,
and bischofite, and the effects of other ions plus temperatures between 25"
and 83OC, confirm this. This method was also applied to determine the
temperature of primary potash deposits. An investigation of the bromide/
sodium chloride relation in salt deposits revealed that bromide can be used
to determine the stratigraphy of evaporite-salt deposits (Baar, 1963).
Derivation of theoretical profiles of bromide thickness versus salt
thickness indicated that, with constant inflow, evaporation, and reflux, the
thickness profiles were all monotomic logarithmic functions. The irregular
and high bromide concentrations of some salt deposits were attributed to
inflow of bromide-rich bitterns from an adjacent potash basin (Holser,
1966).
Shales, sandstones, and carbonates contain about 4, 1, and 6 ppm, respec-
tively, of bromide (Mason, 1966). Sea water contains about 65 mg/l of
bromide, and subsurface petroleum-associated brines contain from less than
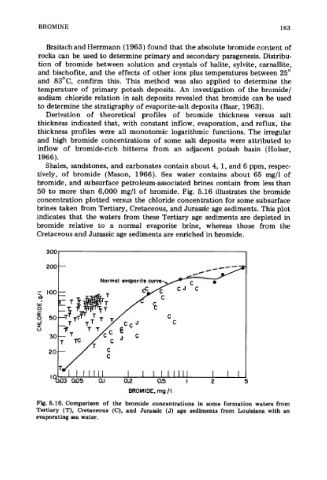
50 to more than 6,000 mg/l of bromide. Fig. 5.16 illustrates the bromide
concentration plotted versus the chloride concentration for some subsurface
brines taken from Tertiary, Cretaceous, and Jurassic age sediments. This plot
indicates that the waters from these Tertiary age sediments are depleted in
bromide relative to a normal evaporite brine, whereas those from the
Cretaceous and Jurassic age sediments are enriched'in bromide.
C
BROMIDE, mg /I
Fig. 5.16. Comparison of the bromide concentrations in some formation waters from
Tertiary (T), Cretaceous (C), and Jurassic (J) age sediments from Louisiana with an
evaporating sea water.