Page 177 - Geochemistry of Oil Field Waters
P. 177
164 INORGANIC CONSTITUENTS AND PHYSICAL PROPERTIES
Normal evaporite curve
P
M I
PI
300 1,000 3000 10
BROMIDE, mgll
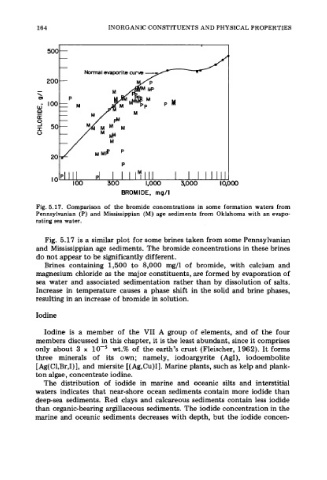
Fig. 5.17. Comparison of the bromide concentrations in some formation waters from
Pennsylvanian (P) and Mississippian (M) age sediments from Oklahoma with an evapo-
rating sea water.
Fig. 5.17 is a similar plot for some brines taken from some Pennsylvanian
and Mississippian age sediments. The bromide concentrations in these brines
do not appear to be significantly different.
Brines containing 1,500 to 8,000 mg/l of bromide, with calcium and
magnesium chloride as the major constituents, are formed by evaporation of
sea water and associated sedimentation rather than by dissolution of salts.
Increase in temperature causes a phase shift in the solid and brine phases,
resulting in an increase of bromide in solution.
Iodine
Iodine is a member of the VII A group of elements, and of the four
members discussed in this chapter, it is the least abundant, since it comprises
only about 3 x lo-' wt.% of the earth's crust (Fleischer, 1962). It forms
three minerals of its own; namely, iodoargyrite (AgI), iodoembolite
[Ag(Cl,Br,I)], and miersite [(Ag,Cu)I]. Marine plants, such as kelp and plank-
ton algae, concentrate iodine.
The distribution of iodide in marine and oceanic silts and interstitial
waters indicates that near-shore ocean Sediments contain more iodide than
deep-sea sediments. Red clays and calcareous sediments contain less iodide
than organic-bearing argillaceous sediments. The iodide concentration in the
marine and oceanic sediments decreases with depth, but the iodide concen-