Page 181 - Geochemistry of Oil Field Waters
P. 181
168 INORGANIC CONSTITUENTS AND PHYSICAL PROPERTIES
1,200 - H2O
000 -
600 1 I
< 400- I /Modern sea water
z I
W 200 - Acid I Alkaline
Fa++ I
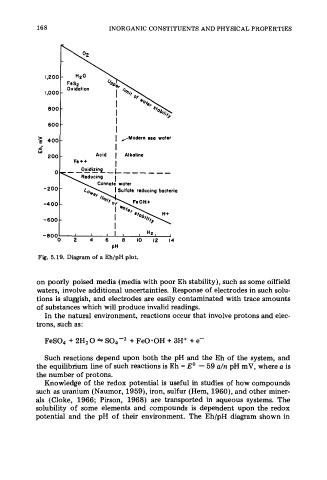
on poorly poised media (media with poor Eh stability), such as some oilfield
waters, involve additional uncertainties. Response of electrodes in such solu-
tions is sluggish, and electrodes are easily contaminated with trace amounts
of substances which will produce invalid readings.
In the natural environment, reactions occur that involve protons and elec-
trons, such as:
FeS04 + 2H2 0 * S04-2 + FeO-OH + 3H+ + e-
Such reactions depend upon both the pH and the Eh of the system, and
the equilibrium line of such reactions is Eh = Eo - 59 a/n pH mV, where a is
the number of protons.
Knowledge of the redox potential is useful in studies of how compounds
such as uranium (Naumor, 1959), iron, sulfur (Hem, 1960), and other miner-
als (Cloke, 1966; Pirson, 1968) are transported in aqueous systems. The
solubility of some elements and compounds is dependent upon the redox
potential and the pH of their environment. The Eh/pH diagram shown in