Page 183 - Geochemistry of Oil Field Waters
P. 183
170 INORGANIC CONSTITUENTS AND PHYSICAL PROPERTIES
pH also may indicate the presence of drilling-mud filtrate or well-treatment
chemicals.
A detailed study indicates that virtually no environment exists on or near
the earth’s surface where the pH/Eh conditions are incompatible with
organic life (Baas Becking et al., 1960). Because COz is the main byproduct
of organic oxidation and the building material of plant and much bacterial
life, it must be expected to play a dominant role. It dissolves in HzO,
producing the bicarbonate ion and a free hydrogen ion. The concentration of
the hydrogen ion is 1 x moles per liter (pH 7) at 25OC in pure water,
but when saturated with COz, it rises to 1 x lo-’ moles per liter (pH 5).
The equilibrium conditions of carbon dioxide, carbonic acid, and the bicar-
bonate ion are:
Hz 0 + COz * Hz CO, * HC03- + H+ * 2H+ + C03-’
and the pH of each equilibrium in ocean water is pH 5, pH 6.3, and pH 10.3.
This reaction moves to the right with increasing temperature in a closed
system. In the presence of organic constituents, the equilibria are modified,
and the pH range can extend from 2 to 12.
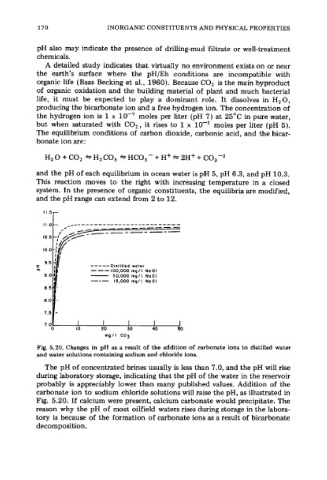
Fig. 5.20. Changes in pH as a result of the addition of carbonate ions to distilled water
and water solutions containing sodium and chloride ions.
The pH of concentrated brines usually is less than 7.0, and the pH will rise
during laboratory storage, indicating that the pH of the water in the reservoir
probably is appreciably lower than many published values. Addition of the
carbonate ion to sodium chloride solutions will raise the pH, as illustrated in
Fig. 5.20. If calcium were present, calcium carbonate would precipitate. The
reason why the pH of most oilfield waters rises during storage in the labora-
tory is because of the formation of carbonate ions as a result of bicarbonate
decomposition.