Page 179 - Geochemistry of Oil Field Waters
P. 179
166 INORGANIC CONSTITUENTS AND PHYSICAL PROPERTIES
1 , 5 0 0 3
1,200 , '
/
~wmi evaporite CUM ,
- I#OOO /-
/
\ /
cn 500 /1
/
w'
2 200
s M P
I loot vs P
20 50E MFP MhP
O t MM
M
M
IODIDE, mg/l
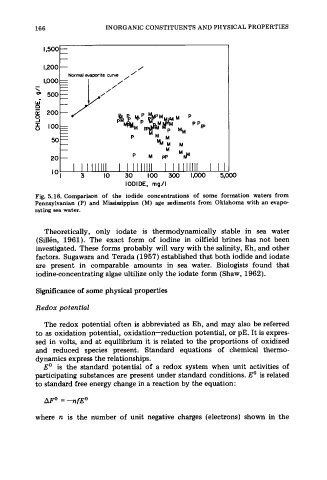
Fig. 5.18. Comparison of the iodide concentrations of some formation waters from
Pennsylvanian (P) and Mississippian (M) age sediments from Oklahoma with an evapo-
rating sea water.
Theoretically, only iodate is thermodynamically stable in sea water
(SillCn, 1961). The exact form of iodine in oilfield brines has not been
investigated. These forms probably will vary with the salinity, Eh, and other
factors. Sugawara and Terada (1957) established that both iodide and iodate
are present in comparable amounts in sea water. Biologists found that
iodine-concentrating algae ultilize only the iodate form (Shaw, 1962).
Significance of some physical properties
Redox potential
The redox potential often is abbreviated as Eh, and may also be referred
to as oxidation potential, oxidation-reduction potential, or pE. It is expres-
sed in volts, and at equilibrium it is related to the proportions of oxidized
and reduced species present. Standard equations of chemical thermo-
dynamics express the relationships.
Eo is the standard potential of a redox system when unit activities of
participating substances are present under standard conditions. Eo is related
to standard free energy change in a reaction by the equation:
where n is the number of unit negative charges (electrons) shown in the