Page 225 - Geochemistry of Oil Field Waters
P. 225
212 ORIGIN OF OILFIELD WATERS
occurs if the equilibria in the aqueous phase remain constant while the water
moves through the sedimentary rocks. Deposition or accumulation of
hydrocarbons occurs if the equilibria in the aqueous phase shifted, causing
desolubilization or precipitation of the hydrocarbons.
Temperature gradients in sedimentary basins usually are about 1°C per 46
m of depth, and the rate of heat flow to the surface is approximately
1.2 x loV6 cal cmV2 sec-I (Birch, 1954). Temperature is believed to be a
primary cause in the conversion of organic matter in rocks to petroleum
(Philippi, 1965), it is also believed that lipids are the major precursors of
petroleum and that most petroleum is generated by chemical reactions oc-
curring at temperatures above 115°C.
Nonmarine sources are recognized for many crude oils, in contrast to the
once general belief that such sources are unfavorable for the generation of
petroleum. Perhaps the best known examples in the United States are the
nonmarine sequences in the Eocene of the Uinta Basin in Utah. Other
examples of oil and gas with continental source sediments are basins such as
the Dzungaria, Tsaidam, Tarim, Turfan, Ordos, Pre-Nan Shan, and Sungliao
of China (Meyerhoff, 1970). There is considerable nonmarine Tertiary age
strata in the Cook Inlet-Kena Basin in coastal Alaska.
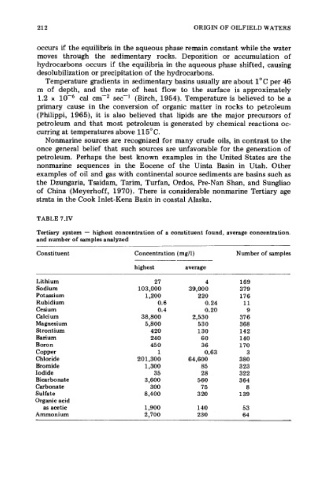
TABLE 7.IV
Tertiary system - highest concentration of a constituent found, average concentration.
and number of samples analyzed
Constituent Concentration (mg/l) Number of samples
highest average
Lithium 27 4 169
Sodium 103,000 39,000 379
Potassium 1,200 2 20 176
Rubidium 0.6 0.24 11
Cesium 0.4 0.20 9
Calcium 38,800 2,530 37 6
Magnesium 5,800 530 368
Strontium 420 130 142
Barium 240 60 140
Boron 450 36 170
Copper 1 0.63 3
Chloride 201,300 64,600 380
Bromide 1,300 85 323
Iodide 35 28 322
Bicarbonate 3,600 560 3 64
Carbonate 300 75 8
Sulfate 8,400 3 20 139
Organic acid
as acetic 1,900 140 53
Ammonium 2,700 230 64