Page 229 - Geochemistry of Oil Field Waters
P. 229
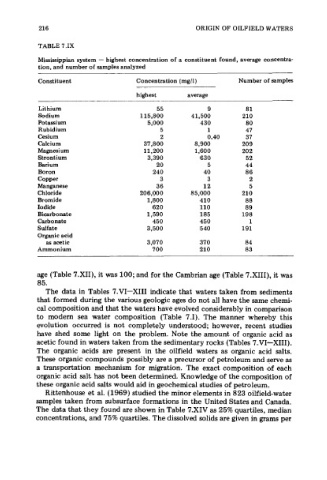
216 ORIGIN OF OILFIELD WATERS
TABLE 7.IX
Mississippian system - highest concentration of a constituent found, average concentra-
tion, and number of samples analyzed
Constituent Concentration (mg/l) Number of samples
highest average
Lithium 55 9 81
Sodium 115,800 41,500 210
Potassium 5,000 430 80
Rubidium 5 1 47
Cesium 2 0.40 37
Calcium 37,800 8,900 209
Magnesium 11,200 1,600 202
Strontium 3,390 630 52
Barium 20 5 44
Boron 240 40 86
Copper 3 3 2
Manganese 36 12 5
Chloride 206,000 85,000 210
Bromide 1,800 410 88
Iodide 620 110 89
Bicarbonate 1,590 185 198
Carbonate 450 450 1
Sulfate 3,500 5 40 191
Organic acid
as acetic 3,070 370 84
Ammonium 700 210 83
age (Table 7.XII), it was 100; and for the Cambrian age (Table 7.X111), it was
85.
The data in Tables 7.VI-XI11 indicate that waters taken from sediments
that formed during the various geologic ages do not all have the same chemi-
cal composition and that the waters have evolved considerably in comparison
to modern sea water composition (Table 7.1). The manner whereby this
evolution occurred is not completely understood; however, recent studies
have shed some light on the problem. Note the amount of organic acid as
acetic found in waters taken from the sedimentary rocks (Tables 7.VI-XIII).
The organic acids are present in the oilfield waters as organic acid salts.
These organic compounds possibly are a precursor of petroleum and serve as
a transportation mechanism for migration. The exact composition of each
organic acid salt has not been determined. Knowledge of the composition of
these organic acid salts would aid in geochemical studies of petroleum.
Rittenhouse et al. (1969) studied the minor elements in 823 oilfield-water
samples taken from subsurface formations in the United States and Canada.
The data that they found are shown in Table 7.XIV as 25% quartiles, median
concentrations, and 75% quartiles. The dissolved solids are given in grams per