Page 232 - Geochemistry of Oil Field Waters
P. 232
RESEARCH STUDIES 21 9
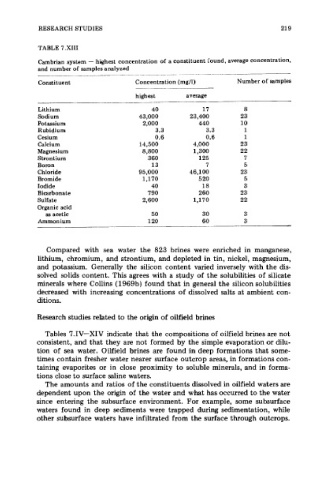
TABLE 7.XIII
Cambrian system - highest concentration of a constituent round, average concentration,
and number of samples analyzed
_______--__ ~- - ~ __
Constituent Concentration (mg/l) Number of samples
highest average
._____ ~___
Lithium 40 17 8
Sodium 43,000 23,400 23
Potassium 2,000 440 10
Rubidium 3.3 3.3 1
Cesium 0.6 0.6 1
Calcium 14,500 4,000 23
Magnesium 8,800 1,300 22
Strontium 3 60 125 7
Boron 13 7 5
Chloride 95,000 46,100 23
Bromide 1,170 520 5
Iodide 40 18 3
Bicarbonate 790 260 23
Sulfate 2,600 1,170 22
Organic acid
as acetic 50 30 3
Ammonium 120 60 3
Compared with sea water the 823 brines were enriched in manganese,
lithium, chromium, and strontium, and depleted in tin, nickel, magnesium,
and potassium. Generally the silicon content varied inversely with the dis-
solved solids content. This agrees with a study of the solubilities of silicate
minerals where Collins (1969b) found that in general the silicon solubilities
decreased with increasing concentrations of dissolved salts at ambient con-
ditions.
Research studies related to the origin of oilfield brines
Tables 7.IV-XIV indicate that the compositions of oilfield brines are not
consistent, and that they are not formed by the simple evaporation or dilu-
tion of sea water. Oilfield brines are found in deep formations that some-
times contain fresher water nearer surface outcrop areas, in formations con-
taining evaporites or in close proximity to soluble minerals, and in forma-
tions close to surface saline waters.
The amounts and ratios of the constituents dissolved in oilfield waters are
dependent upon the origin of the water and what has occurred to the water
since entering the subsurface environment. For example, some subsurface
waters found in deep sediments were trapped during sedimentation, while
other subsurface waters have infiltrated from the surface through outcrops.