Page 243 - Geochemistry of Oil Field Waters
P. 243
RESEARCH STUDIES 227
trations of bromide in many of these brines are lower than the iodide which
is unusual. Localized sedimantary rock deposits enriched in organic iodine
are the source of the high iodide concentrations in these brines (Collins et
al., 1971).
Hot brines
Hot brines containing minor and trace amounts of several metallic
elements in addition to macro concentrations of some alkalies, alkaline
earths, and chloride are found in drill holes in southern California, in the
Caspian Sea, and in deeps in the Red Sea. These brines were formed from
evaporites dissolved by meteoric water, and the metallic elements were
leached from country rocks by the hot brines (Tooms, 1970). Laboratory
reactions of 2M and 4M sodium chloride with andesite and shale at
300"-500°C have produced solutions containing metallic elements in con-
centrations similar to the hot brines (Ellis, 1968).
Comparison of oilfield brines with evaporated sea water
Bromide does not form its own minerals when sea water evaporates. It
forms an isomorphous admixture with chloride in the precipitates
(Valyashko, 1956; Braitsch and Herrmann, 1963). As sea water evaporates,
the carbonates precipitate first, followed by the sulfates. Little or no
bromide precipitates, or if it does, it is occluded with these.
Halite (NaCl) begins to precipitate when the chloride concentration is
about 275,000 mg/l (Table 7.111) compared with that of normal sea water,
19,000 mg/l. Some bromide is entrained with chloride in the precipitate.
2oo t .-
300
c
Normal evaporite curve/
C
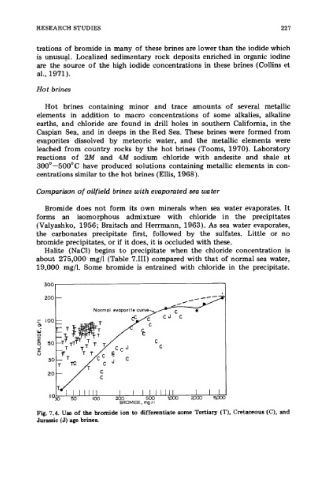
Fig. 7.4. Use of the bromide ion to differentiate some Tertiary (T), Cretaceous (C), and
Jurassic (J) age brines.