Page 248 - Geochemistry of Oil Field Waters
P. 248
23 2 ORIGIN OF OILFIELD WATERS
diagenesis. It has been shown that a tendency exists for potassium to be
adsorbed and fixed by clay minerals, mica, and potassium feldspar in normal
low-temperature processes (White, 1965; Khitarov and Pugin, 1966; Grim,
1952).
The data in Tables 7..III-XIV indicate that the concentration of calcium
in oilfield waters generally is enriched relative to sea water. Cation exchange
reactions with clays accounts for some of this enrichment:
2Na+ (solution) + Ca (clay) + Ca+* (solution) + 2Na (clay)
Collins (1972) found that the ratio Na/(CL + Mg) tends to decrease as the
dissolved solids concentration increases in some oilfield waters from the East
Texas Basin. This depletion of sodium with respect to calcium plus magne-
sium was attributed to diagenesis of the waters and it correlated with an
index of base exchange (Schoeller, 1955), indicating that the alkali metals in
the waters exchanged with alkaline earth metals on the argillaceous minerals
to decrease the dissolved alkali metals and increase the dissolved alkaline
earth metals.
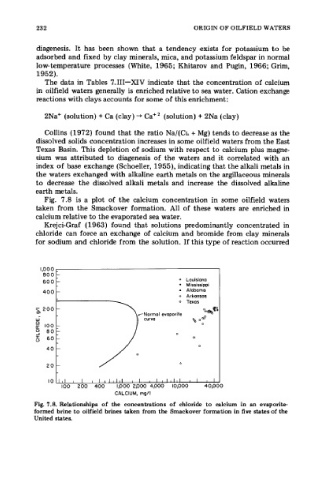
Fig. 7.8 is a plot of the calcium concentration in some oilfield waters
taken from the Smackover formation. All of these waters are enriched in
calcium relative to the evaporated sea water.
Krejci-Graf (1963) found that solutions predominantly concentrated in
chloride can force an exchange of calcium and bromide from clay minerals
for sodium and chloride from the solution. If this type of reaction occurred
3
Fig. 7.8. Relationships of the concentrations of chloride to calcium in an evaporite-
formed brine to oilfield brines taken from the Smackover formation in five states of the
United states.