Page 251 - Geochemistry of Oil Field Waters
P. 251
RESEARCH STUDIES 23 5
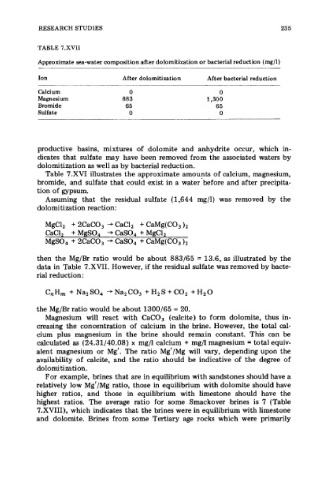
TABLE 7.XVII
Approximate sea-water composition after dolomitization or bacterial reduction (mg/l)
Ion After dolomitization After bacterial reduction
Calcium 0 0
Magnesium 883 1,300
Bromide 65 65
Sulfate 0 0
productive basins, mixtures of dolomite and anhydrite occur, which in-
dicates that sulfate may have been removed from the associated waters by
dolomitization as well as by bacterial reduction.
Table 7.XVI illustrates the approximate amounts of calcium, magnesium,
bromide, and sulfate that could exist in a water’before and after precipita-
tion of gypsum.
Assuming that the residual sulfate (1,644 mg/l) was removed by the
dolomitization reaction:
MgC12 + 2CaC0, += CaC12 + CaMg(C0, )*
--
CaC12- + MgS04- += CaS04 + MgCi2
MgSO, + 2CaC03 + CaS04 + CaMg(C0, )2
then the Mg/Br ratio would be about 883/65 = 13.6, as illustrated by the
data in Table 7.XVII. However, if the residual sulfate was removed by bacte-
rial reduction :
C,H, + Na2 SO4 + Na2C03 + H2S + C02 + H20
the Mg/Br ratio would be about 1300/65 = 20.
Magnesium will react with CaC03 (calcite) to form dolomite, thus in-
creasing the concentration of calcium in the brine. However, the total cal-
cium plus magnesium in the brine should remain constant. This can be
calculated as (24.31/40.08) x mg/l calcium + mg/l magnesium = total equiv-
alent magnesium or Mg’. The ratio Mg’/Mg will vary, depending upon the
availability of calcite, and the ratio should be indicative of the degree of
dolomitization.
For example, brines that are in equilibrium with sandstones should have a
relatively low Mg’/Mg ratio, those in equilibrium with dolomite should have
higher ratios, and those in equilibrium with limestone should have the
highest ratios. The average ratio for some Smackover brines is 7 (Table
7.XVIII), which indicates that the brines were in equilibrium with limestone
and dolomite. Brines from some Tertiary age rocks which were primarily