Page 254 - Geochemistry of Oil Field Waters
P. 254
23 8 ORIGIN OF OILFIELD WATERS
many samples were used in the calculation. For example, 71 Smackover
brines were analyzed for lithium, while 283 were analyzed for sodium.
The concentration ratios (Table 7.XVIII) indicate that all of the deter-
mined constituents in the Smackover brines were enriched with respect to
sea water except sulfate. However, the excess factor ratios indicate that
sodium, potassium, magnesium, chloride, sulfate, and total equivalent mag-
nesium were depleted in the Smackover brines, while lithium, calcium, stron-
tium, barium, copper, iron, manganese, and iodide were enriched. Further,
these ratios indicate that the Smackover brines have been altered consider-
ably if it is assumed that they originally were sea water.
The concentration ratio 48 for bromide (Table 7.XVIII) is one of the
highest that this author has seen. For example, bromide concentration ratios
of 1.2 (Table 7.XIX), 4.4 (Table 7.XX), and 8.8 and 7.2 were found for brines
from Tertiary, Cretaceous, Pennsylvanian, and Mississippian age rocks
(Collins, 1967, 1969a, 1970). The concentration ratios and excess factors in
Tables 7.XIX and XX indicate several constituents are enriched and several
are depleted in these brines also.
Almost one-third of the magnesium in sea water and subsequent bitterns
can be removed during the dolomitization reaction. The formation of
chlorite from montmorillonite requires about 9.2 moles of MgO per mole of
chlorite (Eckhardt, 1958):
1.7 A1203 0.9 MgO 8 Si02 - 2 H20 + 9.2 MgO + 6 H20 +=
10.1 MgO 1.7 A1203 6.4 Si02 * 8 H20 + 1.6 Si02
1,000 -
800 -
600 - a Louisiana
Mississippi
400 - A Alabama
0 Arkansas
\ - 200 - o a */ 0 Texas
cn
W*
g 100:
5 80- ‘Normal evaporite
I 60 - curve
0
40 -
0
20 -
10 III I I I I I I 1161 I I I I I I 1 1 8 1 I I I
00
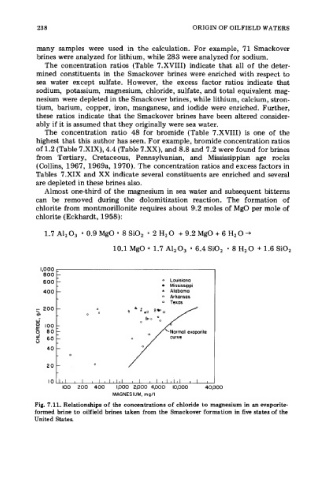
Fig. 7.11. Relationships of the concentrations of chloride to magnesium in an evaporite-
formed brine to oilfield brines taken from the Smackover formation in five states of the
United States.