Page 253 - Geochemistry of Oil Field Waters
P. 253
RESEARCH STUDIES 237
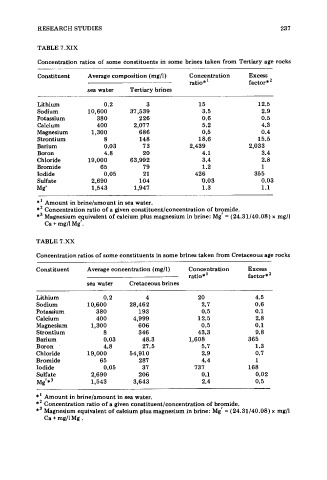
TABLE 7.XIX
Concentration ratios of some constituents in some brines taken from Tertiary age rocks
Constituent Average composition (mg/l) Concentration Excess
ratio*' factor * '
sea water Tertiary brines
Lithium 0.2 3 15 12.5
Sodium 10,600 37,539 3.5 2.9
Potassium 380 226 0.6 0.5
Calcium 400 2,077 5.2 4.3
Magnesium 1,300 686 0.5 0.4
Strontium 8 148 18.6 15.5
Barium 0.03 73 2,439 2,033
Boron 4.8 20 4.1 3.4
Chloride 19,000 63,992 3.4 2.8
Bromide 65 79 1.2 1
Iodide 0.05 21 426 355
Sulfate 2,690 104 0.03 0.03
Mg' 1,543 1,947 1.3 1.1
*' Amount in brine/amount in sea water.
*' Concentration ratio of a given constituent/concentration of brymide.
*3 Magnesium e,quivalent of calcium plus magnesium in brine: Mg = (24.31/40.08) x mg/l
Ca + mg/l Mg .
TABLE 7.XX
Concentration ratios of some constituents in some brines taken from Cretaceous age rocks
Constituent Average concentration (mg/l) Concentration Excess
ratio*' factor*'
sea water Cretaceous brines
Lithium 0.2 4 20 4.5
Sodium 10,600 28,462 2.7 0.6
Potassium 380 193 0.5 0.1
Calcium 400 4,999 12.5 2.8
Magnesium 1,300 606 0.5 0.1
Strontium 8 346 43.3 9.8
Barium 0.03 48.3 1,608 365
Boron 4.8 27.5 5.7 1.3
Chloride 19,000 54,910 2.9 0.7
Bromide 65 287 4.4 1
Iodide 0.05 37 737 168
Sulfate 2,690 206 0.1 0.02
Mg'*3 1,543 3,643 2.4 0.5
*' Amount in brinelamount in sea water.
*' Concentration ratio of a given constituentlconcentration of brymide.
*' Magnesium equivalent of calcium plus magnesium in brine: Mg = (24.31/40.08) x mg/l
Ca + mg/l Mg .