Page 252 - Geochemistry of Oil Field Waters
P. 252
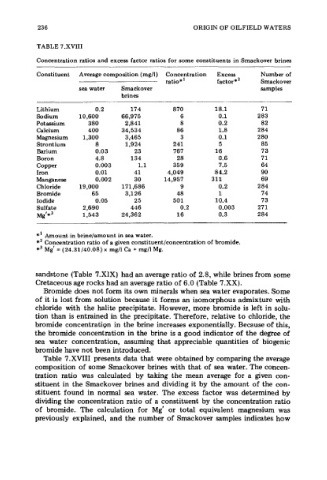
236 ORIGIN OF OILFIELD WATERS
TABLE 7.XVIII
Concentration ratios and excess factor ratios for some constituents in Smackover brines
Constituent Average composition (mg/l) Concentration Excess Number of
ratio*' factor*' Smackover
sea water Smackover samples
brines
Lithium 0.2 17 4 870 18.1 71
Sodium 10,600 66,975 6 0.1 283
Potassium 380 2,841 8 0.2 82
Calcium 400 34,534 86 1.8 284
Magnesium 1,300 3,465 3 0.1 280
Strontium 8 1,924 241 5 85
Barium 0.03 23 767 16 73
Boron 4.8 134 28 0.6 71
Copper 0.003 1.1 359 7.5 64
Iron 0.01 41 4,049 84.2 90
Manganese 0.002 30 14,957 31 1 69
Chloride 19,000 171,686 9 0.2 284
Bromide 65 3,126 48 1 74
Iodide 0.05 25 501 10.4 73
Sulfate 2,690 446 0.2 0.003 27 1
Mg'* 1,543 24,362 16 0.3 284
*' Amount in brine/amount in sea water.
** Concentration ratio of a given constituent/concentration of bromide.
*3 Mg' = (24.31/40.08) x mg/l Ca + mg/l Mg.
sandstone (Table 7.XIX) had an average ratio of 2.8, while brines from some
Cretaceous age rocks had an average ratio of 6.0 (Table 7.XX).
Bromide does not form its own minerals when sea water evaporates. Some
of it is lost from solution because it forms an isomorphous admixture with
chloride with the halite precipitate. However, more bromide is left in solu-
tion than is entrained in the precipitate. Therefore, relative to chloride, the
bromide concentration in the brine increases exponentially. Bemuse of this,
the bromide concentration in the brine is a good indicator of the degree of
sea water concentration, assuming that appreciable quantities of biogenic
bromide have not been introduced.
Table 7.XVIII presents data that were obtained by comparing the average
composition of some Smackover brines with that of sea water. The concen-
tration ratio was calculated by taking the mean average for a given con-
stituent in the Smackover brines and dividing it by the amount of the con-
stituent found in normal sea water. The excess factor was determined by
dividing the concentration ratio of a constituent by the concentration ratio
of bromide. The calculation for Mg' or total equivalent magnesium was
previously explained, and the number of Smackover samples indicates how