Page 249 - Geochemistry of Oil Field Waters
P. 249
RESEARCH STUDIES 233
1,000
000 -
600 - 6 Louisiana
- Mississippi
400 - A Alabama
- 0 Arkansas
Texas
0
- 200 -
\ 0
w
0 100,
5 80
I 60
V
40
20
101I<I I I I I111111 ' I ' I'l'''1 ' I '
100 200 400 1,000 2,000 4,000 10,000 40,000
BROMIDE, mg/l
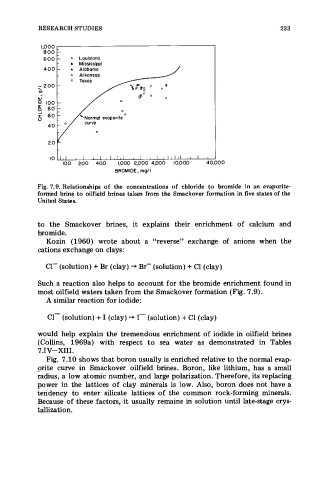
Fig. 7.9. Relationships of the concentrations of chloride to bromide in an evaporite-
formed brine to oilfield brines taken from the Smackover formation in five states of the
United States.
to the Smackover brines, it explains their enrichment of calcium and
bromide.
Kozin (1960) wrote about a "reverse" exchange of anions when the
cations exchange on clays:
C1- (solution) + Br (clay) + Br- (solution) + C1 (clay)
Such a reaction also helps to account for the bromide enrichment found in
most oilfield waters taken from the Smackover formation (Fig. 7.9).
A similar reaction for iodide:
C1- (solution) + I (clay) -, I- (solution) + C1 (clay)
would help explain the tremendous enrichment of iodide in oilfield brines
(Collins, 1969a) with respect to sea water as demonstrated in Tables
7.1 V-XIII.
Fig. 7.10 shows that boron usually is enriched relative to the normal evap-
orite curve in Smackover oilfield brines. Boron, like lithium, has a small
radius, a low atomic number, and large polarization. Therefore, its replacing
power in the lattices of clay minerals is low. Also, boron does not have a
tendency to enter silicate lattices of the common rock-forming minerals.
Because of these factors, it usually remains in solution until late-stage crys-
tallization.