Page 255 - Geochemistry of Oil Field Waters
P. 255
RESEARCH STUDIES 23 9
1,000
000
600 Louisiana
Mississippi
400 Alabama
Arkansas
Texas
- 2 00
\
0
000 4, 0
100 0 0
: 00
2 60 0
0
40 0
0
20
00
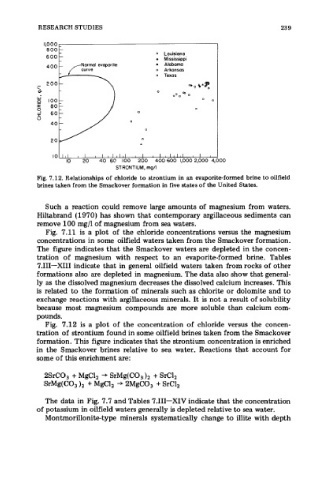
Fig. 7.12. Relationships of chloride to strontium in an evaporite-formed brine to oilfield
brines taken from the Smackover formation in five states of the United States.
Such a reaction could remove large amounts of magnesium from waters.
Hiltabrand (1970) has shown that contemporary argillaceous sediments can
remove 100 mg/l of magnesium from sea waters.
Fig. 7.11 is a plot of the chloride concentrations versus the magnesium
concentrations in some oilfield waters taken from the Smackover formation.
The figure indicates that the Smackover waters are depleted in the concen-
tration of magnesium with respect to an evaporite-formed brine. Tables
7.111-XI11 indicate that in general oilfield waters taken from rocks of other
formations also are depleted in magnesium. The data also show that general-
ly as the dissolved magnesium decreases the dissolved calcium increases. This
is related to the formation of minerals such as chlorite or dolomite and to
exchange reactions with argillaceous minerals. It is not a result of solubility
because most magnesium compounds are more soluble than calcium com-
pounds.
Fig. 7.12 is a plot of the concentration of chloride versus the concen-
tration of strontium found in some oilfield brines taken from the Smackover
formation. This figure indicates that the strontium concentration is enriched
in the Smackover brines relative to sea water. Reactions that account for
some of this enrichment are:
2SrC03 + MgCl, +. SrMg(C03), + SrCl,
SrMg(C0, ), + MgCl, +. 2MgC0, + SrC1,
The data in Fig. 7.7 and Tables 7.111-XIV indicate that the concentration
of potassium in oilfield waters generally is depleted relative to sea water.
Montmorillonite-type minerals systematically change to illite with depth