Page 96 - Geochemistry of Oil Field Waters
P. 96
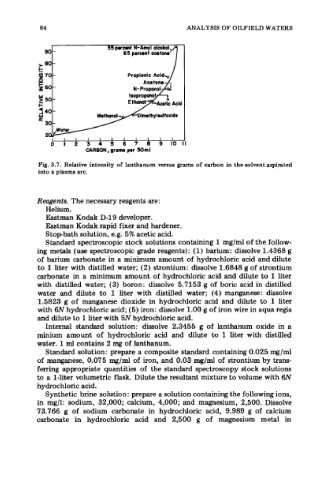
"'i 35 percent N-Amy1 ANALYSIS OF OILFIELD WATERS
84
90
65 percent acetone
Proplonlc Add,
w
Acetone
N-Proponoi
2 50
I-
a 4 30 ~ t h y l ~ l f ~ ~ i d ~
2
I I I I I I I I I I
0 I 2 3 4 5 6 7 8 9 101 I
CARBON, grams per SOml
Fig. 3.7. Relative intensity of lanthanum versus grams of carbon in the solvent aspirated
into a plasma arc.
Reagents. The necessary reagents are:
Helium.
Eastman Kodak D-19 developer.
Eastman Kodak rapid fixer and hardener.
Stop-bath solution, e.g. 5% acetic acid.
Standard spectroscopic stock solutions containing 1 mglml of the follow-
ing metals (use spectroscopic grade reagents): (1) barium: dissolve 1.4368 g
of barium carbonate in a minimum amount of hydrochloric acid and dilute
to 1 liter with distilled water; (2) strontium: dissolve 1.6848 g of strontium
carbonate in a minimum amount of hydrochloric acid and dilute to 1 liter
with distilled water; (3) boron: dissolve 5.7153 g of boric acid in distilled
water and dilute to 1 liter with distilled water; (4) manganese: dissolve
1.5823 g of manganese dioxide in hydrochloric acid and dilute to 1 liter
with 6N hydrochloric acid; (5) iron: dissolve 1.00 g of iron wire in aqua regia
and dilute to 1 liter with 6N hydrochloric acid.
Internal standard solution: dissolve 2.3455 g of lanthanum oxide in a
minium amount of hydrochloric acid and dilute to 1 liter with distilled
water. 1 ml contains 2 mg of lanthanum.
Standard solution: prepare a composite standard containing 0.025 mg/ml
of manganese, 0.075 mg/ml of iron, and 0.03 mg/ml of strontium by trans-
ferring appropriate quantities of the standard spectroscopy stock solutions
to a 1-liter volumetric flask. Dilute the resultant mixture to volume with 6N
hydrochloric acid.
Synthetic brine solution: prepare a solution containing the following ions,
in mg/l: sodium, 32,000; calcium, 4,000; and magnesium, 2,500. Dissolve
73.766 g of sodium carbonate in hydrochloric acid, 9.989 g of calcium
carbonate in hydrochloric acid and 2,500 g of magnesium metal in