Page 205 - Geotechnical Engineering Soil and Foundation Principles and Practice
P. 205
Soil Water
200 Geotechnical Engineering
10.5 THE DIPOLAR NATURE OF WATER
10.5.1 Geometry of a Water Molecule and
Hydrogen Bonding
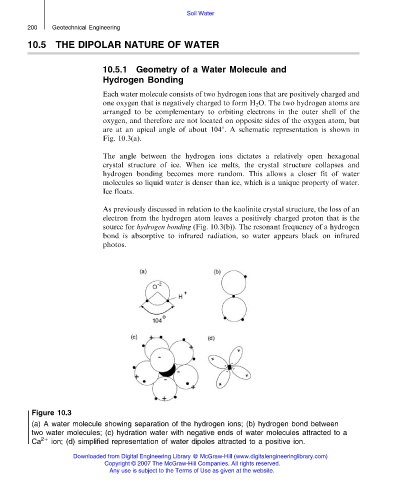
Each water molecule consists of two hydrogen ions that are positively charged and
one oxygen that is negatively charged to form H 2 O. The two hydrogen atoms are
arranged to be complementary to orbiting electrons in the outer shell of the
oxygen, and therefore are not located on opposite sides of the oxygen atom, but
are at an apical angle of about 1048. A schematic representation is shown in
Fig. 10.3(a).
The angle between the hydrogen ions dictates a relatively open hexagonal
crystal structure of ice. When ice melts, the crystal structure collapses and
hydrogen bonding becomes more random. This allows a closer fit of water
molecules so liquid water is denser than ice, which is a unique property of water.
Ice floats.
As previously discussed in relation to the kaolinite crystal structure, the loss of an
electron from the hydrogen atom leaves a positively charged proton that is the
source for hydrogen bonding (Fig. 10.3(b)). The resonant frequency of a hydrogen
bond is absorptive to infrared radiation, so water appears black on infrared
photos.
Figure 10.3
(a) A water molecule showing separation of the hydrogen ions; (b) hydrogen bond between
two water molecules; (c) hydration water with negative ends of water molecules attracted to a
Ca 2þ ion; (d) simplified representation of water dipoles attracted to a positive ion.
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2007 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.