Page 207 - Geotechnical Engineering Soil and Foundation Principles and Practice
P. 207
Soil Water
202 Geotechnical Engineering
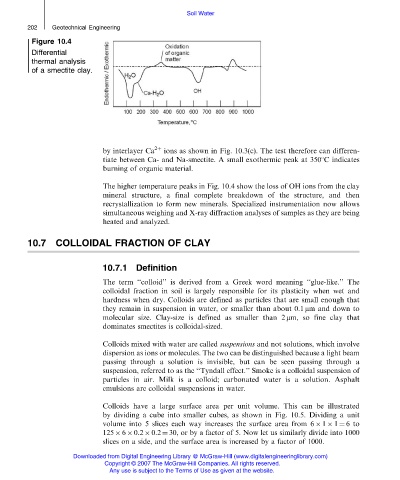
Figure 10.4
Differential
thermal analysis
of a smectite clay.
by interlayer Ca 2þ ions as shown in Fig. 10.3(c). The test therefore can differen-
tiate between Ca- and Na-smectite. A small exothermic peak at 3508C indicates
burning of organic material.
The higher temperature peaks in Fig. 10.4 show the loss of OH ions from the clay
mineral structure, a final complete breakdown of the structure, and then
recrystallization to form new minerals. Specialized instrumentation now allows
simultaneous weighing and X-ray diffraction analyses of samples as they are being
heated and analyzed.
10.7 COLLOIDAL FRACTION OF CLAY
10.7.1 Definition
The term ‘‘colloid’’ is derived from a Greek word meaning ‘‘glue-like.’’ The
colloidal fraction in soil is largely responsible for its plasticity when wet and
hardness when dry. Colloids are defined as particles that are small enough that
they remain in suspension in water, or smaller than about 0.1 mm and down to
molecular size. Clay-size is defined as smaller than 2 mm, so fine clay that
dominates smectites is colloidal-sized.
Colloids mixed with water are called suspensions and not solutions, which involve
dispersion as ions or molecules. The two can be distinguished because a light beam
passing through a solution is invisible, but can be seen passing through a
suspension, referred to as the ‘‘Tyndall effect.’’ Smoke is a colloidal suspension of
particles in air. Milk is a colloid; carbonated water is a solution. Asphalt
emulsions are colloidal suspensions in water.
Colloids have a large surface area per unit volume. This can be illustrated
by dividing a cube into smaller cubes, as shown in Fig. 10.5. Dividing a unit
volume into 5 slices each way increases the surface area from 6 1 1 ¼ 6to
125 6 0.2 0.2 ¼ 30, or by a factor of 5. Now let us similarly divide into 1000
slices on a side, and the surface area is increased by a factor of 1000.
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2007 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.