Page 158 - Geothermal Energy Renewable Energy and The Environment
P. 158
144 Geothermal Energy: Renewable Energy and the Environment
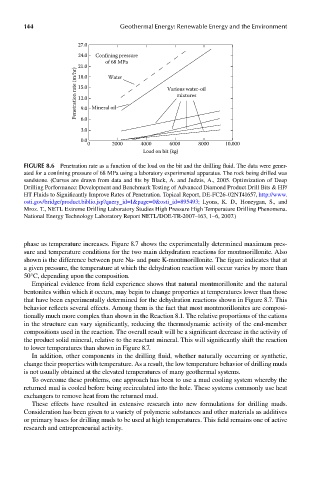
27.0
24.0 Confining pressure
of 68 MPa
Penetration rate (m/hr) 21.0 Water Various water-oil
18.0
15.0
mixtures
12.0
9.0 Mineral oil
6.0
3.0
0.0
0 2000 4000 6000 8000 10,000
Load on bit (kg)
FIGUre 8.6 Penetration rate as a function of the load on the bit and the drilling fluid. The data were gener-
ated for a confining pressure of 68 MPa using a laboratory experimental apparatus. The rock being drilled was
sandstone. (Curves are drawn from data and fits by Black, A. and Judzis, A., 2005. Optimization of Deep
Drilling Performance: Development and Benchmark Testing of Advanced Diamond Product Drill Bits & HP/
HT Fluids to Significantly Improve Rates of Penetration. Topical Report, DE-FC26-02NT41657, http://www.
osti.gov/bridge/product.biblio.jsp?query_id=1&page=0&osti_id=895493; Lyons, K. D., Honeygan, S., and
Mroz. T., NETL Extreme Drilling Laboratory Studies High Pressure High Temperature Drilling Phenomena.
National Energy Technology Laboratory Report NETL/DOE-TR-2007–163, 1–6, 2007.)
phase as temperature increases. Figure 8.7 shows the experimentally determined maximum pres-
sure and temperature conditions for the two main dehydration reactions for montmorillonite. Also
shown is the difference between pure Na- and pure K-montmorillonite. The figure indicates that at
a given pressure, the temperature at which the dehydration reaction will occur varies by more than
50°C, depending upon the composition.
Empirical evidence from field experience shows that natural montmorillonite and the natural
bentonites within which it occurs, may begin to change properties at temperatures lower than those
that have been experimentally determined for the dehydration reactions shown in Figure 8.7. This
behavior reflects several effects. Among them is the fact that most montmorillonites are composi-
tionally much more complex than shown in the Reaction 8.1. The relative proportions of the cations
in the structure can vary significantly, reducing the thermodynamic activity of the end-member
compositions used in the reaction. The overall result will be a significant decrease in the activity of
the product solid mineral, relative to the reactant mineral. This will significantly shift the reaction
to lower temperatures than shown in Figure 8.7.
In addition, other components in the drilling fluid, whether naturally occurring or synthetic,
change their properties with temperature. As a result, the low temperature behavior of drilling muds
is not usually obtained at the elevated temperatures of many geothermal systems.
To overcome these problems, one approach has been to use a mud cooling system whereby the
returned mud is cooled before being recirculated into the hole. These systems commonly use heat
exchangers to remove heat from the returned mud.
These effects have resulted in extensive research into new formulations for drilling muds.
Consideration has been given to a variety of polymeric substances and other materials as additives
or primary bases for drilling muds to be used at high temperatures. This field remains one of active
research and entrepreneurial activity.