Page 237 - Geothermal Energy Renewable Energy and The Environment
P. 237
226 Geothermal Energy: Renewable Energy and the Environment
0.0
–2.0
CO 2 (log moles/kg) –4.0
–6.0
–8.0
0 50 100 150 200 250 300 350
Temperature (°C)
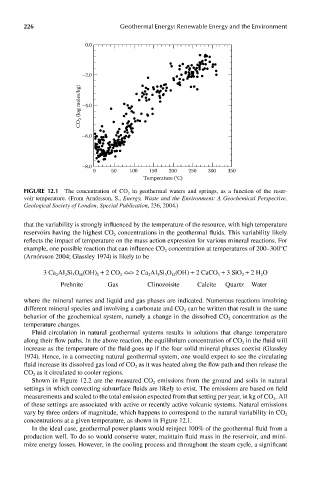
FIGUre 12.1 The concentration of CO 2 in geothermal waters and springs, as a function of the reser-
voir temperature. (From Arnórsson, S., Energy, Waste and the Environment: A Geochemical Perspective,
Geological Society of London, Special Publication, 236, 2004.)
that the variability is strongly influenced by the temperature of the resource, with high temperature
reservoirs having the highest CO concentrations in the geothermal fluids. This variability likely
2
reflects the impact of temperature on the mass action expression for various mineral reactions. For
example, one possible reaction that can influence CO concentration at temperatures of 200–300°C
2
(Arnórsson 2004; Glassley 1974) is likely to be
3 Ca Al Si O (OH) + 2 CO <=> 2 Ca Al Si O (OH) + 2 CaCO + 3 SiO + 2 H O
3
2
2
2
2
3
2
10
2
12
2
3
3
Prehnite Gas Clinozoisite Calcite Quartz Water
where the mineral names and liquid and gas phases are indicated. Numerous reactions involving
different mineral species and involving a carbonate and CO can be written that result in the same
2
behavior of the geochemical system, namely a change in the dissolved CO concentration as the
2
temperature changes.
Fluid circulation in natural geothermal systems results in solutions that change temperature
along their flow paths. In the above reaction, the equilibrium concentration of CO in the fluid will
2
increase as the temperature of the fluid goes up if the four solid mineral phases coexist (Glassley
1974). Hence, in a convecting natural geothermal system, one would expect to see the circulating
fluid increase its dissolved gas load of CO as it was heated along the flow path and then release the
2
CO as it circulated to cooler regions.
2
Shown in Figure 12.2 are the measured CO emissions from the ground and soils in natural
2
settings in which convecting subsurface fluids are likely to exist. The emissions are based on field
measurements and scaled to the total emission expected from that setting per year, in kg of CO . All
2
of these settings are associated with active or recently active volcanic systems. Natural emissions
vary by three orders of magnitude, which happens to correspond to the natural variability in CO
2
concentrations at a given temperature, as shown in Figure 12.1.
In the ideal case, geothermal power plants would reinject 100% of the geothermal fluid from a
production well. To do so would conserve water, maintain fluid mass in the reservoir, and mini-
mize energy losses. However, in the cooling process and throughout the steam cycle, a significant