Page 245 - Geothermal Energy Renewable Energy and The Environment
P. 245
234 Geothermal Energy: Renewable Energy and the Environment
Table 12.2
coefficients of Thermal expansion for Feldspar minerals
mineral α (T ) reference Volume (Å )
−1
3
Microcline a 1.86e−5 722.02
Sanidine b 1.92e−5 723.66
Low Albite a 3.07e−5 664.79
High Albite c 3.15e−5 666.98
a Hovis and Graeme-Barber 1997
b Hovis et al. 1999
c Stewart and von Limbach 1967
∆T = 50° ∆T = 100°
0.005
Sodium
feldspar
0.004 –2,120 cc
Fractional volume change 0.003 –1,640 cc Potassium
0.002
feldspar
0.001 –1,880 cc
–950 cc
0.0
20 40 60 80 100 120 140 160
∆T
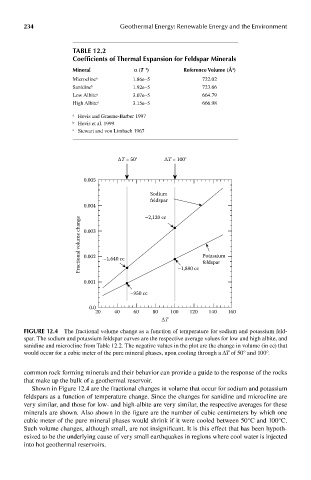
FIGUre 12.4 The fractional volume change as a function of temperature for sodium and potassium feld-
spar. The sodium and potassium feldspar curves are the respective average values for low and high albite, and
sanidine and microcline from Table 12.2. The negative values in the plot are the change in volume (in cc) that
would occur for a cubic meter of the pure mineral phases, upon cooling through a ΔT of 50° and 100°.
common rock forming minerals and their behavior can provide a guide to the response of the rocks
that make up the bulk of a geothermal reservoir.
Shown in Figure 12.4 are the fractional changes in volume that occur for sodium and potassium
feldspars as a function of temperature change. Since the changes for sanidine and microcline are
very similar, and those for low- and high-albite are very similar, the respective averages for these
minerals are shown. Also shown in the figure are the number of cubic centimeters by which one
cubic meter of the pure mineral phases would shrink if it were cooled between 50°C and 100°C.
Such volume changes, although small, are not insignificant. It is this effect that has been hypoth-
esized to be the underlying cause of very small earthquakes in regions where cool water is injected
into hot geothermal reservoirs.