Page 272 - Glucose Monitoring Devices
P. 272
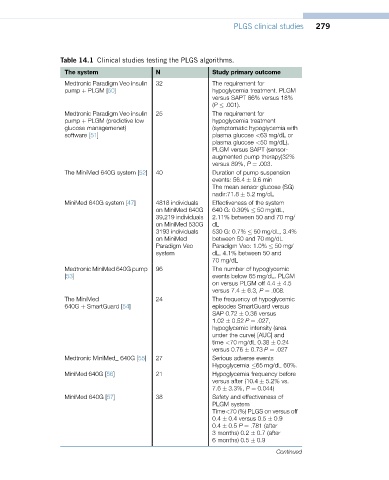
PLGS clinical studies 279
Table 14.1 Clinical studies testing the PLGS algorithms.
The system N Study primary outcome
Medtronic Paradigm Veo insulin 32 The requirement for
pump þ PLGM [50] hypoglycemia treatment. PLGM
versus SAPT 86% versus 18%
(P .001).
Medtronic Paradigm Veo insulin 25 The requirement for
pump þ PLGM (predictive low hypoglycemia treatment
glucose managemenet) (symptomatic hypoglycemia with
software [51] plasma glucose <63 mg/dL or
plasma glucose <50 mg/dL).
PLGM versus SAPT (sensor-
augmented pump therapy)32%
versus 89%, P ¼ .003.
The MiniMed 640G system [52] 40 Duration of pump suspension
events: 56.4 9.6 min
The mean sensor glucose (SG)
nadir:71.8 5.2 mg/dL
MiniMed 640G system [47] 4818 individuals Effectiveness of the system
on MiniMed 640G 640 G: 0.39% 50 mg/dL,
39,219 individuals 2.11% between 50 and 70 mg/
on MiniMed 530G dL
3193 individuals 530 G: 0.7% 50 mg/dL, 3.4%
on MiniMed between 50 and 70 mg/dL
Paradigm Veo Paradigm Veo: 1.0% 50 mg/
system dL, 4.1% between 50 and
70 mg/dL
Medtronic MiniMed 640G pump 96 The number of hypoglycemic
[53] events below 65 mg/dL. PLGM
on versus PLGM off 4.4 4.5
versus 7.4 6.3, P ¼ .008.
The MiniMed 24 The frequency of hypoglycemic
640G þ SmartGuard [54] episodes SmartGuard versus
SAP 0.72 0.36 versus
1.02 0.52 P ¼ .027,
hypoglycemic intensity (area
under the curve) [AUC] and
time <70 mg/dL 0.38 0.24
versus 0.76 0.73 P ¼ .027
Medtronic MiniMed_ 640G [55] 27 Serious adverse events
Hypoglycemia 65 mg/dL 60%.
MiniMed 640G [56] 21 Hypoglycemia frequency before
versus after (10.4 5.2% vs.
7.6 3.3%, P ¼ 0.044)
MiniMed 640G [57] 38 Safety and effectiveness of
PLGM system
Time<70 (%) PLGS on versus off
0.4 0.4 versus 0.5 0.9
0.4 0.5 P ¼ .781 (after
3 months) 0.2 0.7 (after
6 months) 0.5 0.9
Continued