Page 562 - Handbook of Thermal Analysis of Construction Materials
P. 562
532 Chapter 13 - Organic Construction Materials
However, many synthetic macromolecules have a relatively simple struc-
ture being formed by a chemical process called polymerization, which
consists in joining together repeating ( poly) small units (mers) to form a
macromolecule called a polymer.
Polymers with long linear chains and a high degree of symmetry are
called linear (e.g., thermoplastics), and those with side branches are called
branched polymers. If the macromolecules are highly interconnected
through chemical cross-linking of linear polymers by means of chemical
reactions, they become a three-dimensional network (e.g., thermosets).
They are, therefore, called three-dimensional polymers. The mechanical
behavior of cross-linked molecules is different from those without cross-
linking. Examples of polymer types are as follows:
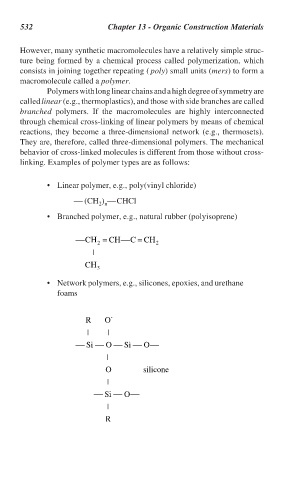
• Linear polymer, e.g., poly(vinyl chloride)
(CH ) CHCl
2 n
• Branched polymer, e.g., natural rubber (polyisoprene)
C Η = CH C = CH
2 2
|
C Η 3
• Network polymers, e.g., silicones, epoxies, and urethane
foams
R O -
| |
Si O Si O
|
O
silicone
|
Si O
|
R