Page 258 - Handbook Of Multiphase Flow Assurance
P. 258
Molecular modeling 257
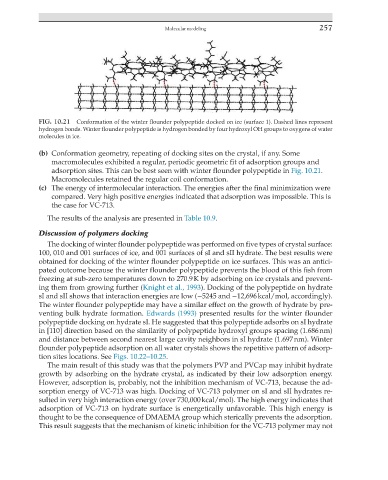
FIG. 10.21 Conformation of the winter flounder polypeptide docked on ice (surface 1). Dashed lines represent
hydrogen bonds. Winter flounder polypeptide is hydrogen bonded by four hydroxyl OH groups to oxygens of water
molecules in ice.
(b) Conformation geometry, repeating of docking sites on the crystal, if any. Some
macromolecules exhibited a regular, periodic geometric fit of adsorption groups and
adsorption sites. This can be best seen with winter flounder polypeptide in Fig. 10.21.
Macromolecules retained the regular coil conformation.
(c) The energy of intermolecular interaction. The energies after the final minimization were
compared. Very high positive energies indicated that adsorption was impossible. This is
the case for VC-713.
The results of the analysis are presented in Table 10.9.
Discussion of polymers docking
The docking of winter flounder polypeptide was performed on five types of crystal surface:
100, 010 and 001 surfaces of ice, and 001 surfaces of sI and sII hydrate. The best results were
obtained for docking of the winter flounder polypeptide on ice surfaces. This was an antici-
pated outcome because the winter flounder polypeptide prevents the blood of this fish from
freezing at sub-zero temperatures down to 270.9 K by adsorbing on ice crystals and prevent-
ing them from growing further (Knight et al., 1993). Docking of the polypeptide on hydrate
sI and sII shows that interaction energies are low (−5245 and −12,696 kcal/mol, accordingly).
The winter flounder polypeptide may have a similar effect on the growth of hydrate by pre-
venting bulk hydrate formation. Edwards (1993) presented results for the winter flounder
polypeptide docking on hydrate sI. He suggested that this polypeptide adsorbs on sI hydrate
in [110] direction based on the similarity of polypeptide hydroxyl groups spacing (1.686 nm)
and distance between second nearest large cavity neighbors in sI hydrate (1.697 nm). Winter
flounder polypeptide adsorption on all water crystals shows the repetitive pattern of adsorp-
tion sites locations. See Figs. 10.22–10.25.
The main result of this study was that the polymers PVP and PVCap may inhibit hydrate
growth by adsorbing on the hydrate crystal, as indicated by their low adsorption energy.
However, adsorption is, probably, not the inhibition mechanism of VC-713, because the ad-
sorption energy of VC-713 was high. Docking of VC-713 polymer on sI and sII hydrates re-
sulted in very high interaction energy (over 730,000 kcal/mol). The high energy indicates that
adsorption of VC-713 on hydrate surface is energetically unfavorable. This high energy is
thought to be the consequence of DMAEMA group which sterically prevents the adsorption.
This result suggests that the mechanism of kinetic inhibition for the VC-713 polymer may not