Page 60 - Handbook Of Multiphase Flow Assurance
P. 60
Fluid characterization 55
1.2 1
Correlation Density at 20°C, g/cm 3 0.8
0.6
0.4
0.2
0
0 0.2 0.4 0.6 0.8 1 1.2
Data Density at 20°C, g/cm 3
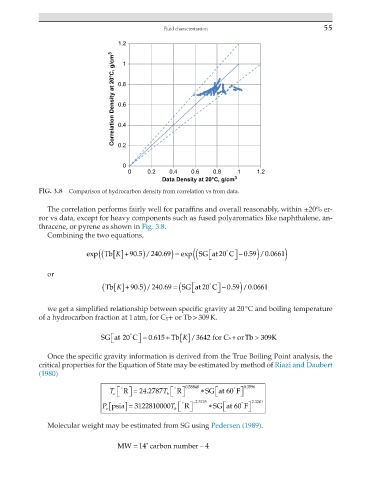
FIG. 3.8 Comparison of hydrocarbon density from correlation vs from data.
The correlation performs fairly well for paraffins and overall reasonably, within ±20% er-
ror vs data, except for heavy components such as fused polyaromatics like naphthalene, an-
thracene, or pyrene as shown in Fig. 3.8.
Combining the two equations,
( (
. )
( (
. / ) 240
69
exp Tb K []+ 90 5 . ) = exp SG at 20 ° C − 059 /.0661 )
0
or
( Tb K []+ 90 5./ = ( SG at 20 ° C − 059 ) 0 0661/.
) 240 69.
.
we get a simplified relationship between specific gravity at 20 °C and boiling temperature
of a hydrocarbon fraction at 1 atm, for C 5 + or Tb > 309 K.
0 615 +
SG at 20 ° C = . Tb[]/ 3642 for C5 + orTb > 30 9K
K
Once the specific gravity information is derived from the True Boiling Point analysis, the
critical properties for the Equation of State may be estimated by method of Riazi and Daubert
(1980)
.
.
°
°
T R = 24 2787. T R 0 58848 ∗SG at 60 ° F 0 3596
c
b
]
.
°
=
P [psia = 3122810000T R −2 3125. ∗SGat 60 ° F 2 3201
c b
Molecular weight may be estimated from SG using Pedersen (1989).
MW = 14 ∗ carbon number − 4