Page 59 - Handbook Of Multiphase Flow Assurance
P. 59
54 3. PVT and rheology investigation
1200
Correlation Boiling Temperature, K 800
1000
600
400
200
0
0 200 400 600 800 1000 1200
Data Boiling Temperature, K
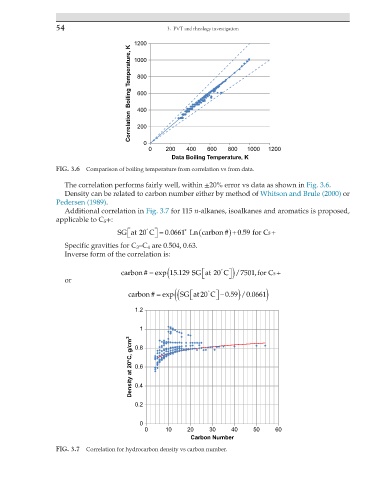
FIG. 3.6 Comparison of boiling temperature from correlation vs from data.
The correlation performs fairly well, within ±20% error vs data as shown in Fig. 3.6.
Density can be related to carbon number either by method of Whitson and Brule (2000) or
Pedersen (1989).
Additional correlation in Fig. 3.7 for 115 n-alkanes, isoalkanes and aromatics is proposed,
applicable to C 5 +:
(
#
SG at 20 ° C = 0 0661 ∗ Lncarbon ) + 059 for C5 +
.
.
Specific gravities for C 3 –C 4 are 0.504, 0.63.
Inverse form of the correlation is:
carbon # = exp( 15 .129 SG at 20 ° C / ) 7501 , for C5 +
or
. )
0
carbon # = exp ( ( SG at 20 ° C − 059 / .0661 )
1.2
1
Density at 20°C, g/cm 3 0.8
0.6
0.4
0.2
0
0 10 20 30 40 50 60
Carbon Number
FIG. 3.7 Correlation for hydrocarbon density vs carbon number.