Page 101 - Handbook of Thermal Analysis of Construction Materials
P. 101
84 Chapter 3 - Formation and Hydration
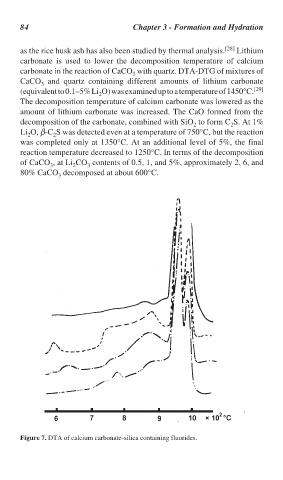
as the rice husk ash has also been studied by thermal analysis. [28] Lithium
carbonate is used to lower the decomposition temperature of calcium
carbonate in the reaction of CaCO with quartz. DTA-DTG of mixtures of
3
CaCO and quartz containing different amounts of lithium carbonate
3
(equivalent to 0.1–5% Li O) was examined up to a temperature of 1450°C. [29]
2
The decomposition temperature of calcium carbonate was lowered as the
amount of lithium carbonate was increased. The CaO formed from the
decomposition of the carbonate, combined with SiO to form C S. At 1%
2
2
Li O, β-C S was detected even at a temperature of 750°C, but the reaction
2
2
was completed only at 1350°C. At an additional level of 5%, the final
reaction temperature decreased to 1250°C. In terms of the decomposition
of CaCO , at Li CO contents of 0.5, 1, and 5%, approximately 2, 6, and
3 2 3
80% CaCO decomposed at about 600°C.
3
Figure 7. DTA of calcium carbonate-silica containing fluorides.