Page 106 - Handbook of Thermal Analysis of Construction Materials
P. 106
Section 6.0 - Hydration 89
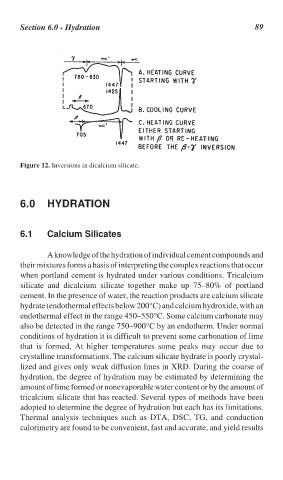
Figure 12. Inversions in dicalcium silicate.
6.0 HYDRATION
6.1 Calcium Silicates
A knowledge of the hydration of individual cement compounds and
their mixtures forms a basis of interpreting the complex reactions that occur
when portland cement is hydrated under various conditions. Tricalcium
silicate and dicalcium silicate together make up 75–80% of portland
cement. In the presence of water, the reaction products are calcium silicate
hydrate (endothermal effects below 200°C) and calcium hydroxide, with an
endothermal effect in the range 450–550°C. Some calcium carbonate may
also be detected in the range 750–900°C by an endotherm. Under normal
conditions of hydration it is difficult to prevent some carbonation of lime
that is formed. At higher temperatures some peaks may occur due to
crystalline transformations. The calcium silicate hydrate is poorly crystal-
lized and gives only weak diffusion lines in XRD. During the course of
hydration, the degree of hydration may be estimated by determining the
amount of lime formed or nonevaporable water content or by the amount of
tricalcium silicate that has reacted. Several types of methods have been
adopted to determine the degree of hydration but each has its limitations.
Thermal analysis techniques such as DTA, DSC, TG, and conduction
calorimetry are found to be convenient, fast and accurate, and yield results