Page 109 - Handbook of Thermal Analysis of Construction Materials
P. 109
92 Chapter 3 - Formation and Hydration
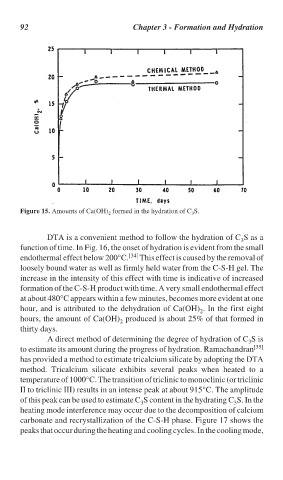
Figure 15. Amounts of Ca(OH) formed in the hydration of C S.
2 3
DTA is a convenient method to follow the hydration of C S as a
3
function of time. In Fig. 16, the onset of hydration is evident from the small
endothermal effect below 200°C. [34] This effect is caused by the removal of
loosely bound water as well as firmly held water from the C-S-H gel. The
increase in the intensity of this effect with time is indicative of increased
formation of the C-S-H product with time. A very small endothermal effect
at about 480°C appears within a few minutes, becomes more evident at one
hour, and is attributed to the dehydration of Ca(OH) . In the first eight
2
hours, the amount of Ca(OH) produced is about 25% of that formed in
2
thirty days.
A direct method of determining the degree of hydration of C S is
3
to estimate its amount during the progress of hydration. Ramachandran [35]
has provided a method to estimate tricalcium silicate by adopting the DTA
method. Tricalcium silicate exhibits several peaks when heated to a
temperature of 1000°C. The transition of triclinic to monoclinic (or triclinic
II to triclinic III) results in an intense peak at about 915°C. The amplitude
of this peak can be used to estimate C S content in the hydrating C S. In the
3
3
heating mode interference may occur due to the decomposition of calcium
carbonate and recrystallization of the C-S-H phase. Figure 17 shows the
peaks that occur during the heating and cooling cycles. In the cooling mode,