Page 105 - Handbook of Thermal Analysis of Construction Materials
P. 105
88 Chapter 3 - Formation and Hydration
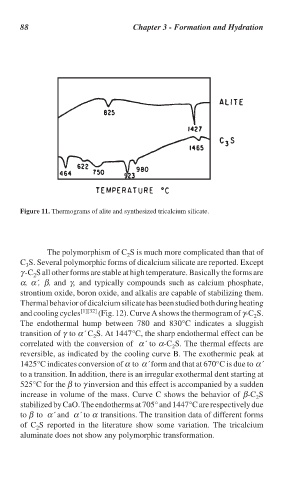
Figure 11. Thermograms of alite and synthesized tricalcium silicate.
The polymorphism of C S is much more complicated than that of
2
C S. Several polymorphic forms of dicalcium silicate are reported. Except
3
γ -C S all other forms are stable at high temperature. Basically the forms are
2
α, α´, β, and γ, and typically compounds such as calcium phosphate,
strontium oxide, boron oxide, and alkalis are capable of stabilizing them.
Thermal behavior of dicalcium silicate has been studied both during heating
and cooling cycles [1][32] (Fig. 12). Curve A shows the thermogram of γ-C S.
2
The endothermal hump between 780 and 830°C indicates a sluggish
transition of γ to α´ C S. At 1447°C, the sharp endothermal effect can be
2
correlated with the conversion of α´ to α-C S. The thermal effects are
2
reversible, as indicated by the cooling curve B. The exothermic peak at
1425°C indicates conversion of α to α´ form and that at 670°C is due to α´
to a transition. In addition, there is an irregular exothermal dent starting at
525°C for the β to γ inversion and this effect is accompanied by a sudden
increase in volume of the mass. Curve C shows the behavior of β-C S
2
stabilized by CaO. The endotherms at 705° and 1447°C are respectively due
to β to α´ and α´ to α transitions. The transition data of different forms
of C S reported in the literature show some variation. The tricalcium
2
aluminate does not show any polymorphic transformation.