Page 93 - Handbook of Thermal Analysis of Construction Materials
P. 93
76 Chapter 3 - Formation and Hydration
siderite, goethite, dolomite, magnesite, gypsum, etc. Thermal methods
have been applied to characterize such minerals. Limestone gives an
endothermal peak at about 800°C for de-carbonation and iron-bearing
limestone gives an additional peak at about 350°C. Dolomite exhibits
two endothermal effects for the decomposition of magnesium carbonate
and calcium carbonate (Fig. 1). DTA technique is ideally suited to
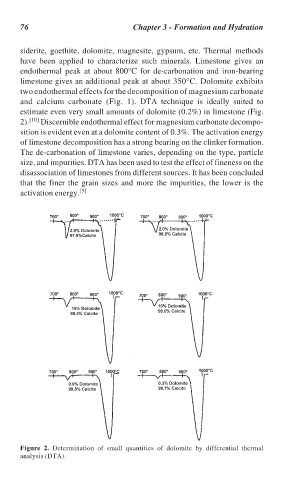
estimate even very small amounts of dolomite (0.2%) in limestone (Fig.
2). [10] Discernible endothermal effect for magnesium carbonate decompo-
sition is evident even at a dolomite content of 0.3%. The activation energy
of limestone decomposition has a strong bearing on the clinker formation.
The de-carbonation of limestone varies, depending on the type, particle
size, and impurities. DTA has been used to test the effect of fineness on the
disassociation of limestones from different sources. It has been concluded
that the finer the grain sizes and more the impurities, the lower is the
activation energy. [5]
Figure 2. Determination of small quantities of dolomite by differential thermal
analysis (DTA).