Page 95 - Handbook of Thermal Analysis of Construction Materials
P. 95
78 Chapter 3 - Formation and Hydration
review of the application of thermal analysis to the clinkerization process
has been published by Courtault. [12]
DTA-TG techniques have been applied to study calcination kinet-
ics of raw materials, quantification of raw materials, determination of total
heat for clinker formation, and prediction of material temperature profile in
a kiln. [14]–[15]
High temperature DTA is a useful tool for studying the clinkering
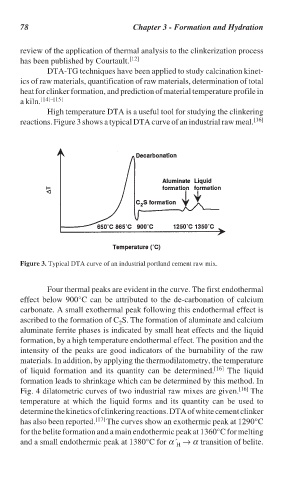
reactions. Figure 3 shows a typical DTA curve of an industrial raw meal. [16]
Figure 3. Typical DTA curve of an industrial portland cement raw mix.
Four thermal peaks are evident in the curve. The first endothermal
effect below 900°C can be attributed to the de-carbonation of calcium
carbonate. A small exothermal peak following this endothermal effect is
ascribed to the formation of C S. The formation of aluminate and calcium
2
aluminate ferrite phases is indicated by small heat effects and the liquid
formation, by a high temperature endothermal effect. The position and the
intensity of the peaks are good indicators of the burnability of the raw
materials. In addition, by applying the thermodilatometry, the temperature
of liquid formation and its quantity can be determined. [16] The liquid
formation leads to shrinkage which can be determined by this method. In
Fig. 4 dilatometric curves of two industrial raw mixes are given. [16] The
temperature at which the liquid forms and its quantity can be used to
determine the kinetics of clinkering reactions. DTA of white cement clinker
has also been reported. [17] The curves show an exothermic peak at 1290°C
for the belite formation and a main endothermic peak at 1360°C for melting
and a small endothermic peak at 1380°C for α´ → α transition of belite.
H