Page 18 - Handbook of Adhesion Promoters
P. 18
2.2 Surface condition and shape 11
Surface roughness is characterized by several methods including arithmetic mean
7
roughness, R , and maximum individual peak-to-valley height, R . These values are
z
a
explained in Figure 2.5.
Mechanical surface treatment such as abrasive action not only affects surface rough-
ness but also removes surface layers which may have different properties then the bulk
8
(and usually do). This may change reactivity of the surface, wetting behavior, and surface
8
energy, and open crevices which are covered by skin.
When polydimethylsiloxane
surface was treated with oxygen
and argon plasma different behav-
9
iors were observed. Oxygen
plasma treatment resulted in for-
mation of hydroxyl groups and
surface roughness whereas only
surface roughness was changed in
the case of argon plasma treat-
9
ment. Oxygen plasma treatment
gave only slightly higher peel
strength than was the case of argon
9
plasma treatment.
In still another study, the sur-
face roughness was induced to
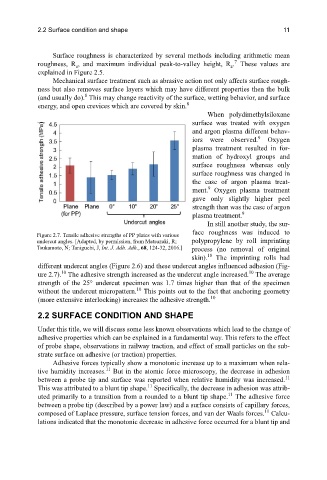
Figure 2.7. Tensile adhesive strengths of PP plates with various
undercut angles. [Adapted, by permission, from Matsuzaki, R; polypropylene by roll imprinting
Tsukamoto, N; Taniguchi, J, Int. J. Adh. Adh., 68, 124-32, 2016.] process (no removal of original
10
skin). The imprinting rolls had
different undercut angles (Figure 2.6) and these undercut angles influenced adhesion (Fig-
10
10
ure 2.7). The adhesive strength increased as the undercut angle increased. The average
strength of the 25° undercut specimen was 1.7 times higher than that of the specimen
10
without the undercut micropattern. This points out to the fact that anchoring geometry
10
(more extensive interlocking) increases the adhesive strength.
2.2 SURFACE CONDITION AND SHAPE
Under this title, we will discuss some less known observations which lead to the change of
adhesive properties which can be explained in a fundamental way. This refers to the effect
of probe shape, observations in railway traction, and effect of small particles on the sub-
strate surface on adhesive (or traction) properties.
Adhesive forces typically show a monotonic increase up to a maximum when rela-
11
tive humidity increases. But in the atomic force microscopy, the decrease in adhesion
between a probe tip and surface was reported when relative humidity was increased. 11
11
This was attributed to a blunt tip shape. Specifically, the decrease in adhesion was attrib-
11
uted primarily to a transition from a rounded to a blunt tip shape. The adhesive force
between a probe tip (described by a power law) and a surface consists of capillary forces,
11
composed of Laplace pressure, surface tension forces, and van der Waals forces. Calcu-
lations indicated that the monotonic decrease in adhesive force occurred for a blunt tip and