Page 25 - Handbook of Adhesion Promoters
P. 25
18 Mechanisms of Adhesion
tion of phosphorylcholine polymer containing cationic amino groups, poly[2-methacry-
26
loyloxyethyl phosphorylcholine (MPC)-co-2-aminoethylmethacrylate]. The cationic
phosphorylcholine polymer adsorbed on the anionic membrane surface prevents bacterial
26
adhesion and solves the problem of reduced performance.
Microbial adhesion is initiated by passive adsorption via attractive physicochemical
27
interactions (e.g., van der Waals and electrostatic forces). At high ionic concentrations,
27
van der Waals interactions are predominant. At low ionic concentrations, the adhesive
27
forces are increased with increasing electrostatic attraction. The electrostatic interactions
27
contribute to microbial adhesive forces on solid surfaces.
Chemically crosslinked poly(allylamine hydrochloride)-dextran adhesive films can
load negatively charged drugs such as ibuprofen based on the electrostatic interaction
between microgel and ibuprofen molecules and release them under some physiological
conditions (e.g., change of ionic
28
concentration). Adhesive films
based on electrostatic interaction
can be designed to bond/debond
multiple times by an externally
28
applied electric field.
Unmodified silica nanoparti-
cles were used to prepare a poly-
mer-based nanocomposite with
improved interfacial adhesion and
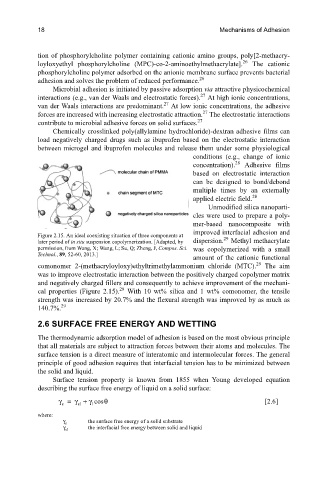
Figure 2.15. An ideal coexisting situation of three components at 29
later period of in situ suspension copolymerization. [Adapted, by dispersion. Methyl methacrylate
permission, from Wang, X; Wang, L; Su, Q; Zheng, J, Compos. Sci. was copolymerized with a small
Technol., 89, 52-60, 2013.]
amount of the cationic functional
29
comonomer 2-(methacryloyloxy)ethyltrimethylammonium chloride (MTC). The aim
was to improve electrostatic interaction between the positively charged copolymer matrix
and negatively charged fillers and consequently to achieve improvement of the mechani-
29
cal properties (Figure 2.15). With 10 wt% silica and 1 wt% comonomer, the tensile
strength was increased by 20.7% and the flexural strength was improved by as much as
29
140.7%.
2.6 SURFACE FREE ENERGY AND WETTING
The thermodynamic adsorption model of adhesion is based on the most obvious principle
that all materials are subject to attraction forces between their atoms and molecules. The
surface tension is a direct measure of interatomic and intermolecular forces. The general
principle of good adhesion requires that interfacial tension has to be minimized between
the solid and liquid.
Surface tension property is known from 1855 when Young developed equation
describing the surface free energy of liquid on a solid surface:
γ = γ + γ cos θ [2.6]
s
sl
l
where:
the surface free energy of a solid substrate
γ s
the interfacial free energy between solid and liquid
γ sl