Page 27 - Handbook of Adhesion Promoters
P. 27
20 Mechanisms of Adhesion
In epoxy to aluminum adhesion, the surface energy played a leading role in improve-
ment of adhesive bonding when full wetting was achieved, whereas surface roughness
34
affected adhesion strength when wetting was incomplete.
The adhesion force was measured by atomic force microscopy to analyze the adhe-
35
sion energy between the graphene and the substrate. The graphene/SiO , graphene/gold,
2
2
and graphene/graphene adhesion energies per surface area were 270, 255, and 307 mJ/m ,
35
respectively.
Atmospheric plasma and pyrolysis of ethanol improve the tensile strength and inter-
36
facial adhesion of carbon fibers. Coating of only several nanometers gives fiber the
higher surface energy and better wettability and provides a strong mechanical interlocking
36
with the epoxy matrix. The interfacial shear strength of epoxy composites containing
36
modified fibers increases by 27.9%.
The ice adhesion strength to the sili-
cone-based hydrophobic surfaces was ~43
times lower than to the bare polished alu-
minum alloy indicating excellent anti-icing
37
property of these coatings. Superhydro-
phobic coatings displayed poor anti-icing
property in spite of their high water repel-
37
lence. A smooth surfaces with a low sur-
face energy promote a low ice adhesion
37
strength.
Many of the above examples show
that adhesion is promoted by a combination
of surface roughness and surface energy,
and their influences are frequently quite
difficult to separate. It is not surprising that
these effects are interfering with each other
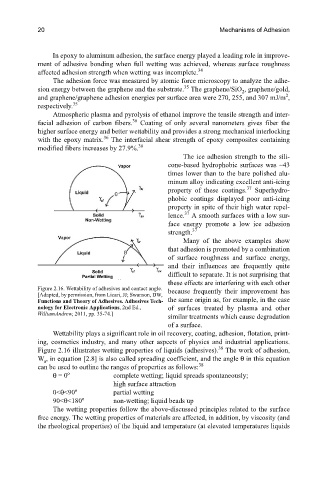
Figure 2.16. Wettability of adhesives and contact angle. because frequently their improvement has
[Adapted, by permission, from Licari, JJ; Swanson, DW,
Functions and Theory of Adhesives. Adhesives Tech- the same origin as, for example, in the case
nology for Electronic Applications, 2nd Ed., of surfaces treated by plasma and other
WilliamAndrew, 2011, pp. 35-74.] similar treatments which cause degradation
of a surface.
Wettability plays a significant role in oil recovery, coating, adhesion, flotation, print-
ing, cosmetics industry, and many other aspects of physics and industrial applications.
38
Figure 2.16 illustrates wetting properties of liquids (adhesives). The work of adhesion,
W , in equation [2.8] is also called spreading coefficient, and the angle θ in this equation
a
38
can be used to outline the ranges of properties as follows:
θ = 0 o complete wetting; liquid spreads spontaneously;
high surface attraction
0<θ<90 o partial wetting
90<θ<180 o non-wetting; liquid beads up
The wetting properties follow the above-discussed principles related to the surface
free energy. The wetting properties of materials are affected, in addition, by viscosity (and
the rheological properties) of the liquid and temperature (at elevated temperatures liquids