Page 28 - Handbook of Adhesion Promoters
P. 28
2.6 Surface free energy and wetting 21
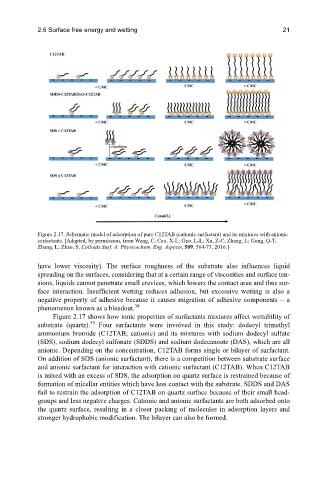
Figure 2.17. Schematic model of adsorption of pure C12TAB (cationic surfactant) and its mixtures with anionic
surfactants. [Adapted, by permission, from Wang, C; Cao, X-L; Guo, L-L; Xu, Z-C; Zhang, L; Gong, Q-T;
Zhang, L; Zhao, S, Colloids Surf. A: Physicochem. Eng. Aspects, 509, 564-73, 2016.]
have lower viscosity). The surface roughness of the substrate also influences liquid
spreading on the surfaces, considering that at a certain range of viscosities and surface ten-
sions, liquids cannot penetrate small crevices, which lowers the contact area and thus sur-
face interaction. Insufficient wetting reduces adhesion, but excessive wetting is also a
negative property of adhesive because it causes migration of adhesive components − a
38
phenomenon known as a bleedout.
Figure 2.17 shows how ionic properties of surfactants mixtures affect wettability of
39
substrate (quartz). Four surfactants were involved in this study: dodecyl trimethyl
ammonium bromide (C12TAB; cationic) and its mixtures with sodium dodecyl sulfate
(SDS), sodium dodecyl sulfonate (SDDS) and sodium dodecanoate (DAS), which are all
anionic. Depending on the concentration, C12TAB forms single or bilayer of surfactant.
On addition of SDS (anionic surfactant), there is a competition between substrate surface
and anionic surfactant for interaction with cationic surfactant (C12TAB). When C12TAB
is mixed with an excess of SDS, the adsorption on quartz surface is restrained because of
formation of micellar entities which have less contact with the substrate. SDDS and DAS
fail to restrain the adsorption of C12TAB on quartz surface because of their small head-
groups and less negative charges. Cationic and anionic surfactants are both adsorbed onto
the quartz surface, resulting in a closer packing of molecules in adsorption layers and
stronger hydrophobic modification. The bilayer can also be formed.