Page 138 - Handbook of Battery Materials
P. 138
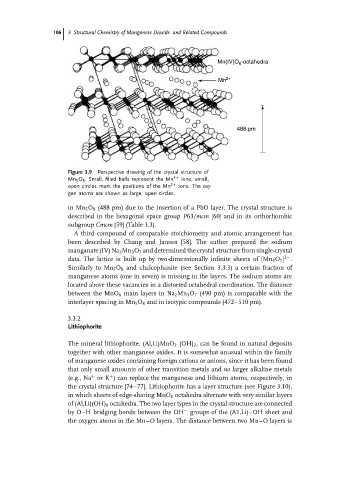
106 3 Structural Chemistry of Manganese Dioxide and Related Compounds
Mn(IV)O -octahedra
6
Mn 2+
488 pm
Figure 3.9 Perspective drawing of the crystal structure of
Mn 5 O 8 . Small, filled balls represent the Mn 4+ ions; small,
open circles mark the positions of the Mn 2+ ions. The oxy-
gen atoms are shown as large, open circles.
in Mn 5 O 8 (488 pm) due to the insertion of a PbO layer. The crystal structure is
described in the hexagonal space group P63/mcm [60] and in its orthorhombic
subgroup Cmcm [59] (Table 3.3).
A third compound of comparable stoichiometry and atomic arrangement has
been described by Chang and Jansen [58]. The author prepared the sodium
manganate (IV) Na 2 Mn 3 O 7 and determined the crystal structure from single-crystal
2−
data. The lattice is built up by two-dimensionally infinite sheets of [Mn 3 O 7 ] .
Similarly to Mn 5 O 8 and chalcophanite (see Section 3.3.3) a certain fraction of
manganese atoms (one in seven) is missing in the layers. The sodium atoms are
located above these vacancies in a distorted octahedral coordination. The distance
between the MnO 6 main layers in Na 2 Mn 3 O 7 (490 pm) is comparable with the
interlayer spacing in Mn 5 O 8 and in isotypic compounds (472–510 pm).
3.3.2
Lithiophorite
The mineral lithiophorite, (Al,Li)MnO 2 (OH) 2 , can be found in natural deposits
together with other manganese oxides. It is somewhat unusual within the family
of manganese oxides containing foreign cations or anions, since it has been found
that only small amounts of other transition metals and no larger alkaline metals
+
(e.g., Na or K ) can replace the manganese and lithium atoms, respectively, in
+
the crystal structure [74–77]. Lithiophorite has a layer structure (see Figure 3.10),
in which sheets of edge-sharing MnO 6 octahedra alternate with very similar layers
of (Al,Li)(OH) 6 octahedra. The two layer types in the crystal structure are connected
by O–H bridging bonds between the OH groups of the (A1,Li)–OH sheet and
−
the oxygen atoms in the Mn–O layers. The distance between two Mn–O layers is