Page 139 - Handbook of Battery Materials
P. 139
3.3 Layer Structures 107
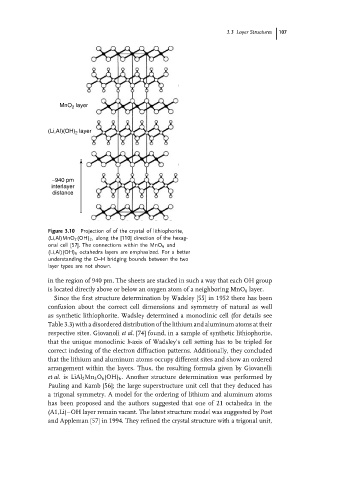
MnO layer
2
(Li,AI)(OH) 2 layer
∼940 pm
interlayer
distance
Figure 3.10 Projection of of the crystal of lithiophorite,
(Li,Al)MnO 2 (OH) 2 , along the [110] direction of the hexag-
onal cell [57]. The connections within the MnO 6 and
(Li,Al)(OH) 6 octahedra layers are emphasized. For a better
understanding the O–H bridging bounds between the two
layer types are not shown.
in the region of 940 pm. The sheets are stacked in such a way that each OH group
is located directly above or below an oxygen atom of a neighboring MnO 6 layer.
Since the first structure determination by Wadsley [55] in 1952 there has been
confusion about the correct cell dimensions and symmetry of natural as well
as synthetic lithiophorite. Wadsley determined a monoclinic cell (for details see
Table 3.3) with a disordered distribution of the lithium and aluminum atoms at their
respective sites. Giovanoli et al. [74] found, in a sample of synthetic lithiophorite,
that the unique monoclinic b-axis of Wadsley’s cell setting has to be tripled for
correct indexing of the electron diffraction patterns. Additionally, they concluded
that the lithium and aluminum atoms occupy different sites and show an ordered
arrangement within the layers. Thus, the resulting formula given by Giovanelli
et al.isLiAl 2 Mn 3 O 6 (OH) 6 . Another structure determination was performed by
Pauling and Kamb [56]; the large superstructure unit cell that they deduced has
a trigonal symmetry. A model for the ordering of lithium and aluminum atoms
has been proposed and the authors suggested that one of 21 octahedra in the
(A1,Li)–OH layer remain vacant. The latest structure model was suggested by Post
and Appleman [57] in 1994. They refined the crystal structure with a trigonal unit,