Page 144 - Handbook of Battery Materials
P. 144
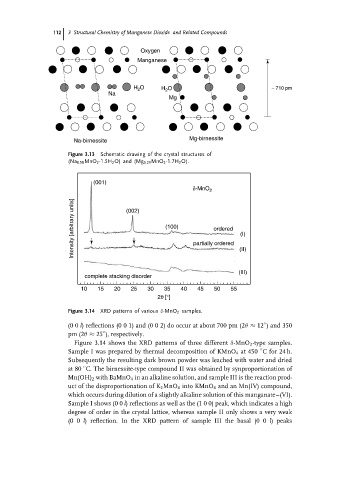
112 3 Structural Chemistry of Manganese Dioxide and Related Compounds
Oxygen
Manganese
H O H O ∼ 710 pm
2
2
Na
Mg
Na-birnessite Mg-birnessite
Figure 3.13 Schematic drawing of the crystal structures of
(Na 0.58 MnO 2 ·1.5H 2 O) and (Mg 0.29 MnO 2 ·1.7H 2 O).
(001)
δ-MnO 2
Intensity [arbitrary units] (002) (100) partially ordered (II)
ordered
(I)
(III)
complete stacking disorder
10 15 20 25 30 35 40 45 50 55
2θ [°]
Figure 3.14 XRD patterns of various δ-MnO 2 samples.
◦
(0 0 l) reflections (0 0 1) and (0 0 2) do occur at about 700 pm (2θ ≈ 12 ) and 350
◦
pm (2θ ≈ 25 ), respectively.
Figure 3.14 shows the XRD patterns of three different δ-MnO 2 -type samples.
◦
Sample I was prepared by thermal decomposition of KMnO 4 at 450 Cfor 24 h.
Subsequently the resulting dark brown powder was leached with water and dried
at 80 C. The birnessite-type compound II was obtained by synproportionation of
◦
Mn(OH) 2 with BaMnO 4 in an alkaline solution, and sample III is the reaction prod-
uct of the disproportionation of K 2 MnO 4 into KMnO 4 and an Mn(IV) compound,
which occurs during dilution of a slightly alkaline solution of this manganate–(VI).
Sample I shows (0 0 l) reflections as well as the (1 0 0) peak, which indicates a high
degree of order in the crystal lattice, whereas sample II only shows a very weak
(0 0 l) reflection. In the XRD pattern of sample III the basal (0 0 l)peaks