Page 140 - Handbook of Battery Materials
P. 140
108 3 Structural Chemistry of Manganese Dioxide and Related Compounds
which has a similar c-axis (c = 2816.9 pm) to the cell proposed by Pauling et al.
(2820 pm), but a very short a-axis of only 282.5 pm. In the structural model of
Post and Appleman the lithium and aluminum atoms are statistically distributed
at their respective crystallographic sites (this model is shown in Figure 3.10).
All the structure models discussed above describe generally the same structural
feature: the alternation of Mn–O and (Al,Li)–OH sheets. The only difference is
that each model deals with a slightly different atomic arrangement in terms of
ordering at the Al/Li site and the commensurability of the layer stacking in the
various superstructures of the simple monoclinic model proposed by Wadsley.
Since all of these authors investigated different samples with possibly slightly
differing structural properties, none of the models is necessarily to be preferred.
3.3.3
Chalcophanite
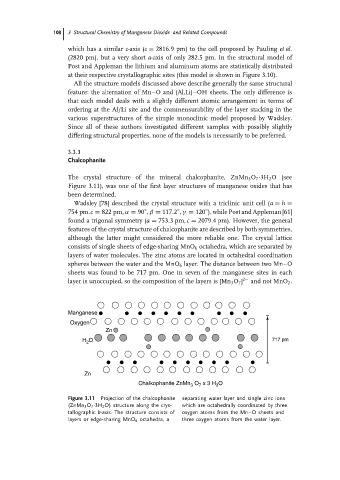
The crystal structure of the mineral chalcophanite, ZnMn 3 O 7 ·3H 2 O(see
Figure 3.11), was one of the first layer structures of manganese oxides that has
been determined.
Wadsley [78] described the crystal structure with a triclinic unit cell (a = h =
◦
◦
754 pm.c = 822 pm, α = 90 , β = 117.2 , γ = 120 ), while Post and Appleman [61]
◦
found a trigonal symmetry (a = 753.3 pm, c = 2079.4 pm). However, the general
features of the crystal structure of chalcophanite are described by both symmetries,
although the latter might considered the more reliable one. The crystal lattice
consists of single sheets of edge-sharing MnO 6 octahedra, which are separated by
layers of water molecules. The zinc atoms are located in octahedral coordination
spheres between the water and the MnO 6 layer. The distance between two Mn–O
sheets was found to be 717 pm. One in seven of the manganese sites in each
layer is unoccupied, so the composition of the layers is [Mn 3 O 7 ] 2− and not MnO 2 .
Manganese
Oxygen
Zn
O 717 pm
H 2
Zn
O x 3 H O
Chalkophanite ZnMn 3 7 3
Figure 3.11 Projection of the chalcophanite separating water layer and single zinc ions
(ZnMn 3 O 7 ·3H 2 O) structure along the crys- which are octahedrally coordinated by three
tallographic b-axis. The structure consists of oxygen atoms from the Mn–O sheets and
layers or edge-sharing MnO 6 octahedra, a three oxygen atoms from the water layer.