Page 150 - Handbook of Battery Materials
P. 150
118 3 Structural Chemistry of Manganese Dioxide and Related Compounds
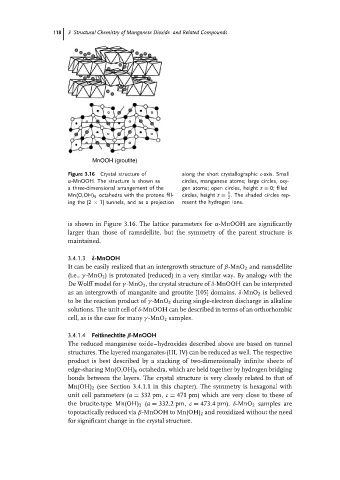
MnOOH (groutite)
Figure 3.16 Crystal structure of along the short crystallographic c-axis. Small
α-MnOOH. The structure is shown as circles, manganese atoms; large circles, oxy-
a three-dimensional arrangement of the gen atoms; open circles, height z = 0; filled
1
Mn(O,OH) 6 octahedra with the protons fill- circles, height z = . The shaded circles rep-
2
ing the [2 × 1] tunnels, and as a projection resent the hydrogen ions.
is shown in Figure 3.16. The lattice parameters for α-MnOOH are significantly
larger than those of ramsdellite, but the symmetry of the parent structure is
maintained.
3.4.1.3 δ-MnOOH
It can be easily realized that an intergrowth structure of β-MnO 2 and ramsdellite
(i.e., γ -MnO 2 ) is protonated (reduced) in a very similar way. By analogy with the
De Wolff model for γ -MnO 2 , the crystal structure of δ-MnOOH can be interpreted
as an intergrowth of manganite and groutite [105] domains. δ-MnO 2 is believed
to be the reaction product of γ -MnO 2 during single-electron discharge in alkaline
solutions. The unit cell of δ-MnOOH can be described in terms of an orthorhombic
cell, as is the case for many γ -MnO 2 samples.
3.4.1.4 Feitknechtite β-MnOOH
The reduced manganese oxide–hydroxides described above are based on tunnel
structures. The layered manganates-(III, IV) can be reduced as well. The respective
product is best described by a stacking of two-dimensionally infinite sheets of
edge-sharing Mn(O,OH) 6 octahedra, which are held together by hydrogen bridging
bonds between the layers. The crystal structure is very closely related to that of
Mn(OH) 2 (see Section 3.4.1.1 in this chapter). The symmetry is hexagonal with
unit cell parameters (a = 332 pm, c = 471 pm) which are very close to these of
the brucite-type Mn(OH) 2 (a = 332.2pm, c = 473.4 pm). δ-MnO 2 samples are
topotactically reduced via β-MnOOH to Mn(OH) 2 and reoxidized without the need
for significant change in the crystal structure.