Page 204 - Handbook of Battery Materials
P. 204
6.3 The Thermodynamic Situation 173
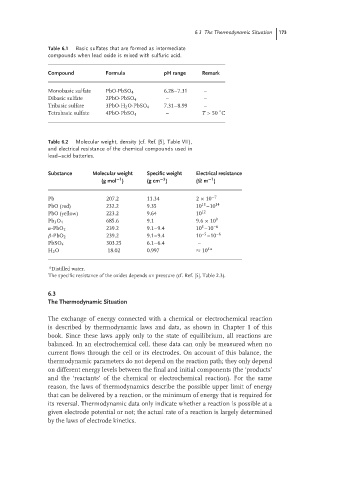
Table 6.1 Basic sulfates that are formed as intermediate
compounds when lead oxide is mixed with sulfuric acid.
Compound Formula pH range Remark
Monobasic sulfate PbO·PbSO 4 6.28–7.31 –
Dibasic sulfate 2PbO·PbSO 4 – –
Tribasic sulfate 3PbO·H 2 O·PbSO 4 7.31–8.99 –
◦
Tetrabasic sulfate 4PbO·PbSO 4 – T > 50 C
Table 6.2 Molecular weight, density (cf. Ref. [5], Table VII),
and electrical resistance of the chemical compounds used in
lead–acid batteries.
Substance Molecular weight Specific weight Electrical resistance
−3
−1
−1
(g mol ) (g cm ) (Ω m )
Pb 207.2 11.34 2 × 10 −7
13
PbO (red) 232.2 9.35 10 –10 14
PbO (yellow) 223.2 9.64 10 12
Pb 3 O 4 685.6 9.1 9.6 × 10 9
5
α-PbO 2 239.2 9.1–9.4 10 –10 –6
–5
β-PbO 2 239.2 9.1–9.4 10 –10 –6
303.25 6.1–6.4 –
PbSO 4
H 2 O 18.02 0.997 ≈ 10 4a
a
Distilled water.
The specific resistance of the oxides depends on pressure (cf. Ref. [5], Table 2.3).
6.3
The Thermodynamic Situation
The exchange of energy connected with a chemical or electrochemical reaction
is described by thermodynamic laws and data, as shown in Chapter 1 of this
book. Since these laws apply only to the state of equilibrium, all reactions are
balanced. In an electrochemical cell, these data can only be measured when no
current flows through the cell or its electrodes. On account of this balance, the
thermodynamic parameters do not depend on the reaction path; they only depend
on different energy levels between the final and initial components (the ‘products’
and the ‘reactants’ of the chemical or electrochemical reaction). For the same
reason, the laws of thermodynamics describe the possible upper limit of energy
that can be delivered by a reaction, or the minimum of energy that is required for
its reversal. Thermodynamic data only indicate whether a reaction is possible at a
given electrode potential or not; the actual rate of a reaction is largely determined
by the laws of electrode kinetics.