Page 211 - Handbook of Battery Materials
P. 211
180 6 Lead Oxides
Fortunately, the kinetic parameters reduce the rates of these reactions so far that
the gradual self-discharge of the PbO 2 is such a slow reaction that it usually does
not affect the performance of the battery.
• Self-discharge by oxygen generation (Equation 6.25) occurs equivalent to a current
in the range of 0.5 mA/100 Ah, which means ∼0.4% of nominal capacity per
month (starting at higher values).
• Corrosion of the positive grid (Equation 6.28) occurs equivalent to about
1 mA/100 Ah at open-circuit voltage and intact passivation layer. It depends
on electrode potential, and is at minimum about 40–80 mV above the
PbSO 4 /PbO 2 equilibrium potential. The corrosion rate depends furthermore
to some extent on alloy composition and is increased with high-antimony
alloys.
• As already mentioned, hydrogen oxidation can be neglected.
Although the rate of these reactions is slow, according to its thermodynamic
situation the lead dioxide electrode is not stable. Since a similar situation applies
to the negative electrode, the lead–acid battery system as a whole is unstable and a
certain rate of water decomposition cannot be avoided.
6.3.4
Thermodynamic Data
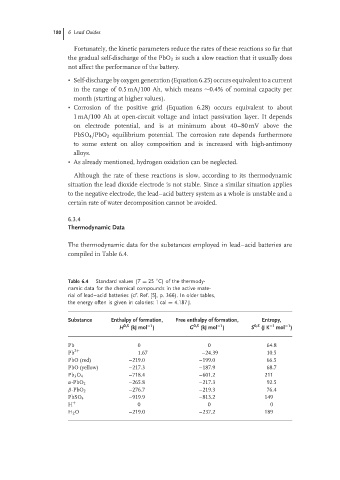
The thermodynamic data for the substances employed in lead–acid batteries are
compiled in Table 6.4.
◦
Table 6.4 Standard values (T = 25 C) of the thermody-
namic data for the chemical compounds in the active mate-
rial of lead–acid batteries (cf. Ref. [5], p. 366). In older tables,
the energy often is given in calories: 1 cal = 4.187 J.
Substance Enthalpy of formation, Free enthalpy of formation, Entropy,
−1
−1
−1
H 0,S (kJ mol ) G 0,S (kJ mol ) S 0,S (J K −1 mol )
Pb 0 0 64.8
Pb 2+ 1.67 –24.39 10.5
PbO (red) –219.0 –199.0 66.5
PbO (yellow) –217.3 –187.9 68.7
Pb 3 O 4 –718.4 –601.2 211
α-PbO 2 –265.8 –217.3 92.5
β-PbO 2 –276.7 –219.3 76.4
PbSO 4 –919.9 –813.2 149
H + 0 0 0
H 2 O –219.0 –237.2 189