Page 245 - Handbook of Battery Materials
P. 245
214 7 Bromine-Storage Materials
Fe/Cu
Al
PP 8% 3%
PE/C 2%
8%
PE Electrolyte
17% 61%
Viton
1%
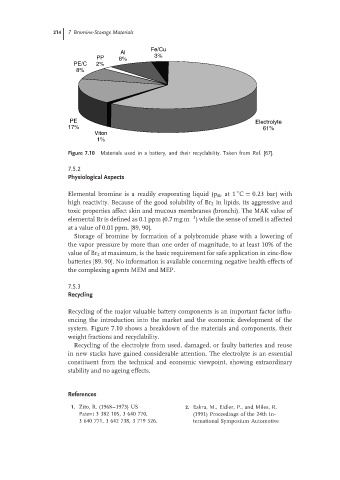
Figure 7.10 Materials used in a battery, and their recyclability. Taken from Ref. [67].
7.5.2
Physiological Aspects
◦
Elemental bromine is a readily evaporating liquid (p Br at 1 C = 0.23 bar) with
high reactivity. Because of the good solubility of Br 2 in lipids, its aggressive and
toxic properties affect skin and mucous membranes (bronchi). The MAK value of
−3
elemental Br is defined as 0.1 ppm (0.7 mg m ) while the sense of smell is affected
at a value of 0.01 ppm. [89, 90].
Storage of bromine by formation of a polybromide phase with a lowering of
the vapor pressure by more than one order of magnitude, to at least 10% of the
value of Br 2 at maximum, is the basic requirement for safe application in zinc-flow
batteries [89, 90]. No information is available concerning negative health effects of
the complexing agents MEM and MEP.
7.5.3
Recycling
Recycling of the major valuable battery components is an important factor influ-
encing the introduction into the market and the economic development of the
system. Figure 7.10 shows a breakdown of the materials and components, their
weight fractions and recyclability.
Recycling of the electrolyte from used, damaged, or faulty batteries and reuse
in new stacks have gained considerable attention. The electrolyte is an essential
constituent from the technical and economic viewpoint, showing extraordinary
stability and no ageing effects.
References
1. Zito, R. (1968–1973) US 2. Eskra, M., Eidler, P., and Miles, R.
Patent 3 382 105, 3 640 770, (1991) Proceedings of the 24th In-
3 640 771, 3 642 738, 3 719 526. ternational Symposium Automotive