Page 241 - Handbook of Battery Materials
P. 241
210 7 Bromine-Storage Materials
chloro compounds N-chloromethyl-N-methyl morpholidinium bromide (C-MEM)
◦
and C-MEP, white precipitates were formed at 0 C upon mixing with an aqueous
solution of 1 mol L −1 ZnBr 2 . Symmetrically substituted QBr compounds such as
+
+
Me 4 N Br − and Et 4 N Br − yielded solid polybromide phases over wide ranges
of temperature and composition [48]. A pronounced influence not only of the
temperature but also of the bromine contents of the complex phases is reviewed.
Results for the corresponding aqueous phases of the electrolyte can be found in
the original paper, which also contains studies of a number of electrolyte phases
made up of MEM:MEP mixtures.
7.4
Analytical Study of a Battery Charge Cycle
In order to improve the bromine-storing capacity and hence the battery
efficiency of a zinc-flow cell, knowledge of the structure and consistency of
the complex phase during the entire charge–discharge cycle is an essential
requirement.
Until today the only available data, obtained by direct sampling of a prototype
battery system concerning mass flow of the complexing agents as well as the
Br 2 produced in both the aqueous and nonaqueous electrolyte phases, have been
gained by application of Raman spectroscopy [87, 88].
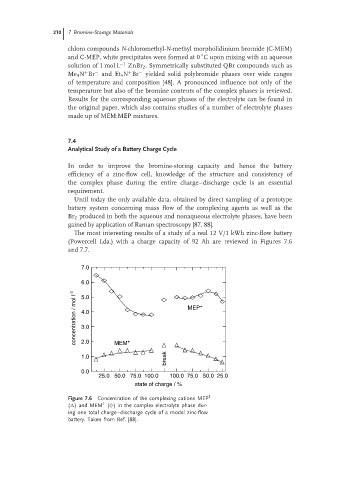
The most interesting results of a study of a real 12 V/1 kWh zinc-flow battery
(Powercell Lda.) with a charge capacity of 92 Ah are reviewed in Figures 7.6
and 7.7.
7.0
6.0
concentration / mol l -1 4.0 MEP +
5.0
3.0
2.0
break
1.0 MEM +
0.0
25.0 50.0 75.0 100.0 100.0 75.0 50.0 25.0
state of charge / %
Figure 7.6 Concentration of the complexing cations MEP 1
1
( ) and MEM (♦) in the complex electrolyte phase dur-
ing one total charge–discharge cycle of a model zinc-flow
battery. Taken from Ref. [88].