Page 240 - Handbook of Battery Materials
P. 240
7.3 Physical Properties of the Bromine Storage Phase 209
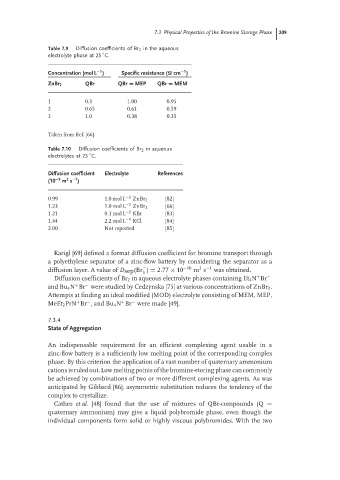
Table 7.9 Diffusion coefficients of Br 2 in the aqueous
◦
electrolyte phase at 25 C.
−1
−1
Concentration (mol L ) Specific resistance (Ω cm )
ZnBr 2 QBr QBr = MEP QBr = MEM
1 0.3 1.00 0.95
2 0.65 0.61 0.59
3 1.0 0.38 0.35
Taken from Ref. [66].
Table 7.10 Diffusion coefficients of Br 2 in aqueous
◦
electrolytes at 25 C.
Diffusion coefficient Electrolyte References
–1
2
(10 −9 m s )
0.99 1.0 mol L −1 ZnBr 2 [82]
1.23 1.0 mol L −1 ZnBr 2 [66]
1.21 0.1 mol L −1 KBr [83]
1.44 2.2 mol L −1 KCl [84]
2.00 Not reported [85]
Karigl [69] defined a format diffusion coefficient for bromine transport through
a polyethylene separator of a zinc-flow battery by considering the separator as a
2
diffusion layer. A value of Dsep (Br ) = 2.77 × 10 −10 m s −1 was obtained.
−
3
Diffusion coefficients of Br 2 in aqueous electrolyte phases containing Et 4 N Br −
+
+
−
and Bu 4 N Br were studied by Cedzynska [75] at various concentrations of ZnBr 2 .
Attempts at finding an ideal modified (MOD) electrolyte consisting of MEM, MEP,
+
−
MeEt 2 PrN Br , and Bu 4 N Br were made [49].
+
−
7.3.4
State of Aggregation
An indispensable requirement for an efficient complexing agent usable in a
zinc-flow battery is a sufficiently low melting point of the corresponding complex
phase. By this criterion the application of a vast number of quaternary ammonium
cations is ruled out. Low melting points of the bromine-storing phase can commonly
be achieved by combinations of two or more different complexing agents. As was
anticipated by Gibbard [86], asymmetric substitution reduces the tendency of the
complex to crystallize.
Cathro et al. [48] found that the use of mixtures of QBr-compounds (Q =
quaternary ammonium) may give a liquid polybromide phase, even though the
individual components form solid or highly viscous polybromides. With the two